New preparation method of gadobenate dimeglumine
A technology of gadobenate meglumine and its synthesis method, which is applied in the field of synthesis research of nuclear magnetic resonance contrast agents, can solve problems affecting the purity of the final product, a large amount of hydrochloric acid, and low product purity, and is beneficial to the control of product quality and reaction conditions Simple, quality control and stable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] "Example 1" Preparation of 2-chloro-3-benzyloxypropionic acid potassium salt (III)
[0042] In a 1L three-necked reaction flask, at room temperature, add 389g of benzyl alcohol and 0.83g of sodium metal, stir for 10 minutes, then add 105g of 2-chloroacrylonitrile dropwise in an ice bath. Continue to react in the bath for 1 hour, then stir and react at room temperature for 2 hours, add 300ml of ethyl acetate, and dropwise add 200ml of dilute hydrochloric acid with a content of 10%. 10% NaHCO 3 The solution was washed, washed with water, dried over anhydrous magnesium sulfate, filtered to remove the desiccant, and the filtrate was concentrated to obtain a dark red oil.
[0043]Add the oil to 20% sodium hydroxide (360ml) solution, stir at 50°C for 30min, add 500ml of water, extract with ethyl acetate (500ml×2), adjust the pH of the aqueous layer to 2 with concentrated hydrochloric acid, extract with ethyl acetate (500ml×3), combined, dried with anhydrous sulfuric acid, f...
Embodiment 2
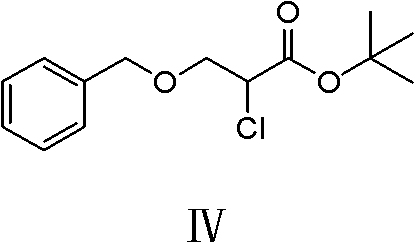
[0044] "Example 2": Preparation of tert-butyl 2-chloro-3-benzyloxypropionate (IV)
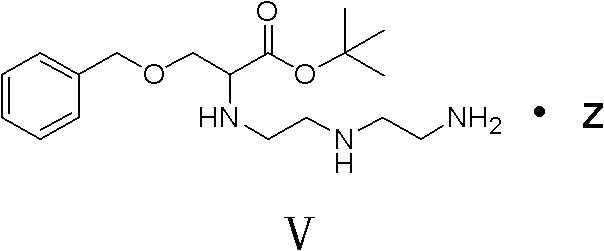
[0045] In a 1L reaction flask, 42.8g of 2-chloro-3-benzyloxypropionic acid was dissolved in 200ml of acetonitrile, under ice cooling, the temperature was controlled at 0-5°C, 29.6g of tert-butanol and 2.44g of DMAP were added, Add 45.3 g of DCC in batches. After the addition, keep the reaction in ice bath for 30 minutes, and react at room temperature for 8 hours, filter, concentrate the filtrate until a yellow oily substance is obtained, separate by column, use petroleum ether as eluent, separate and purify, concentrate and wash The liquid was removed to obtain 43 g of a colorless oily substance, namely tert-butyl 2-chloro-3-benzyloxypropionate, with a yield of 86% (calculated according to 2-chloro-3-benzyloxypropionic acid). "Example 3" Preparation of 2-(diethylenetriamino)-3-benzyloxy propionate tert-butyl fumarate (V)
Embodiment 3
[0045] In a 1L reaction flask, 42.8g of 2-chloro-3-benzyloxypropionic acid was dissolved in 200ml of acetonitrile, under ice cooling, the temperature was controlled at 0-5°C, 29.6g of tert-butanol and 2.44g of DMAP were added, Add 45.3 g of DCC in batches. After the addition, keep the reaction in ice bath for 30 minutes, and react at room temperature for 8 hours, filter, concentrate the filtrate until a yellow oily substance is obtained, separate by column, use petroleum ether as eluent, separate and purify, concentrate and wash The liquid was removed to obtain 43 g of a colorless oily substance, namely tert-butyl 2-chloro-3-benzyloxypropionate, with a yield of 86% (calculated according to 2-chloro-3-benzyloxypropionic acid). "Example 3" Preparation of 2-(diethylenetriamino)-3-benzyloxy propionate tert-butyl fumarate (V)
[0046] In a 500ml reaction flask, dissolve 76g of diethylenetriamine in 80ml of DMF, and slowly add 40g of tert-butyl 2-chloro-3-benzyloxypropionate and 40m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com