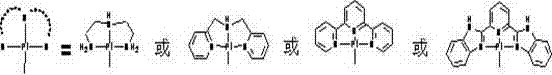
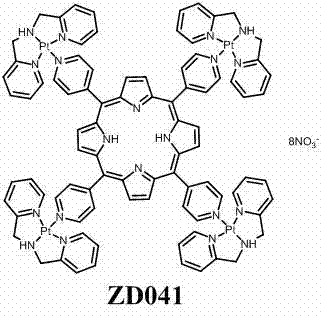
Tetrapyridylporphine bridged crossed tetra-palladium complexes, and preparation method and antitumor activity thereof
A technology of tetrapyridyl porphyrin bridge and pyridyl porphyrin bridge, which is applied in the field of preparation of tetranuclear platinum complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0031] Preparation of the cross-shaped tetranuclear platinum complex of embodiment 1 tetrapyridyl porphyrin bridge:
[0032] Dissolve 0.40 millimoles of DPA platinum (II) complex in 15 milliliters of water, add 0.40 millimoles of silver nitrate in a dark place under nitrogen protection, stir at 60°C for 36 hours, and centrifuge to discard the precipitate after the reaction is complete. Keep the clear solution. Dissolve 0.10 mmoles of tetrakis(4-pyridyl)porphyrin in 10 ml of trifluoroethanol, and add it dropwise to the above clear liquid. After the addition is complete, add 10 ml of trifluoroethanol to ensure that no turbidity occurs . The reaction solution was reacted at 60°C in the dark for 2 days under the protection of nitrogen. After the reaction was completed, the reaction solution was concentrated under reduced pressure to 5 ml, and an appropriate amount of absolute ethanol and absolute ether were added to immediately precipitate a purple solid, which was centrifuged t...
Embodiment 2
[0034] Example 2 Antitumor activity experiment of tetrapyridyl porphyrin-bridged cruciform tetranuclear platinum complexes:
[0035] Adopt the compound prepared in embodiment 1 to carry out following test:
[0036] Cell lines and culture conditions: The cell lines used in this experiment are as follows: human colorectal carcinoma cells (HCT-8), human gastric adenocarcinoma cells (BGC-823), human melanoma carcinoma cells (A375), human nasopharyngeal carcinoma cells (KB) and human rectal cancer cells (HT-29). The cells were cultured in DMEM medium containing 10% fetal bovine serum, which contained 100 units of penicillin and 100 micrograms of streptomycin per milliliter, and the cells were seeded in a 10 cm diameter petri dish at 37 degrees, 5% CO 2 The cells were cultured in the environment, and passaged by trypsinization when the cells were confluent.
[0037] Cytotoxicity test: Cytotoxicity was measured by MTT method. The cells were digested with 0.25% trypsin into a singl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com