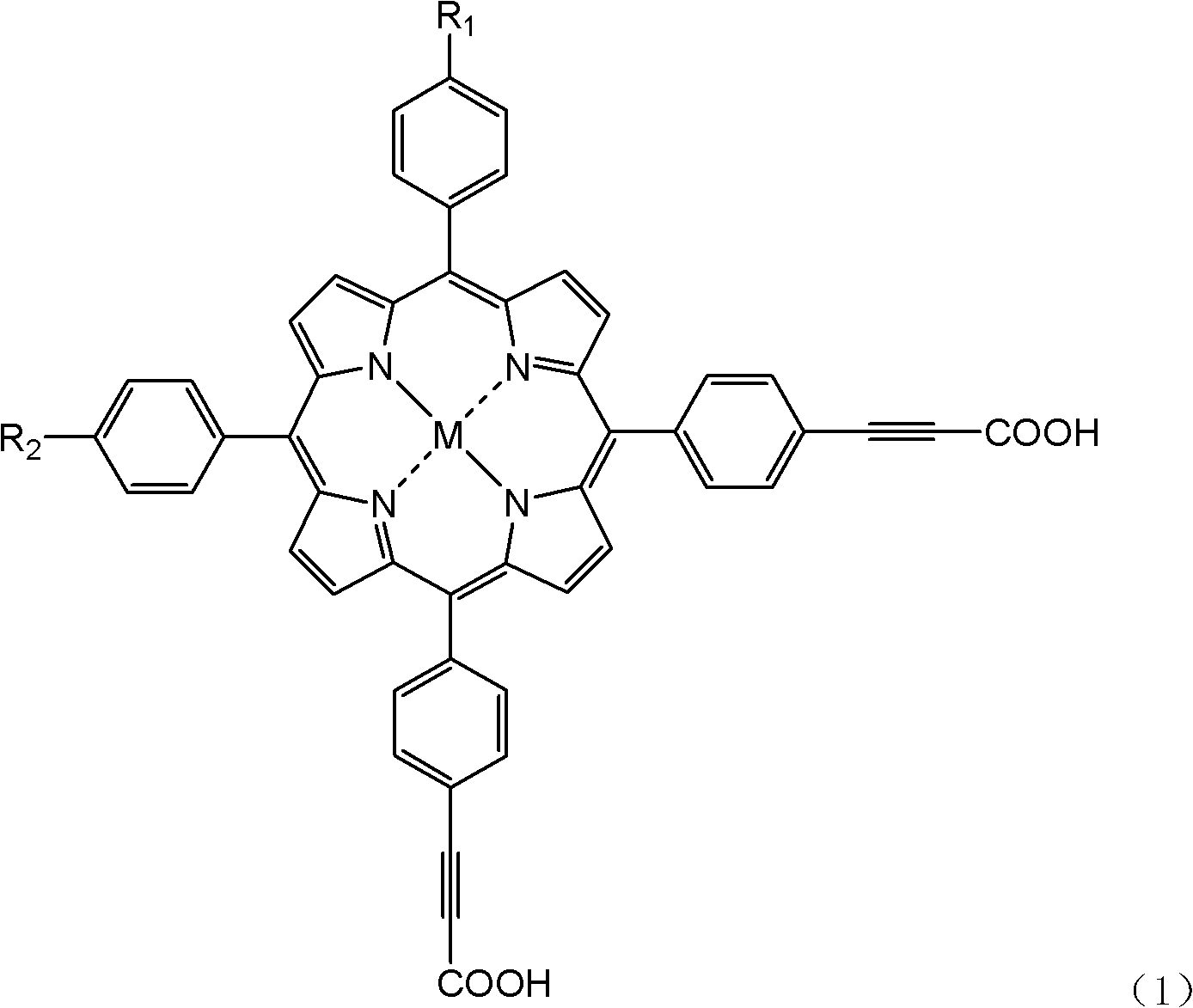
Asymmetrical dye molecule adopting tetraphenylporphin as core, and preparation method thereof
A tetraphenylporphyrin and asymmetric technology, which is applied in the field of preparation of porphyrin dye molecules, can solve the problems that the photoelectric conversion efficiency of porphyrin-based batteries is not as good as that, affects the performance of photovoltaic cells, and the molecules are easy to aggregate, etc. Electron and electron-withdrawing ability, conversion efficiency improvement, and low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
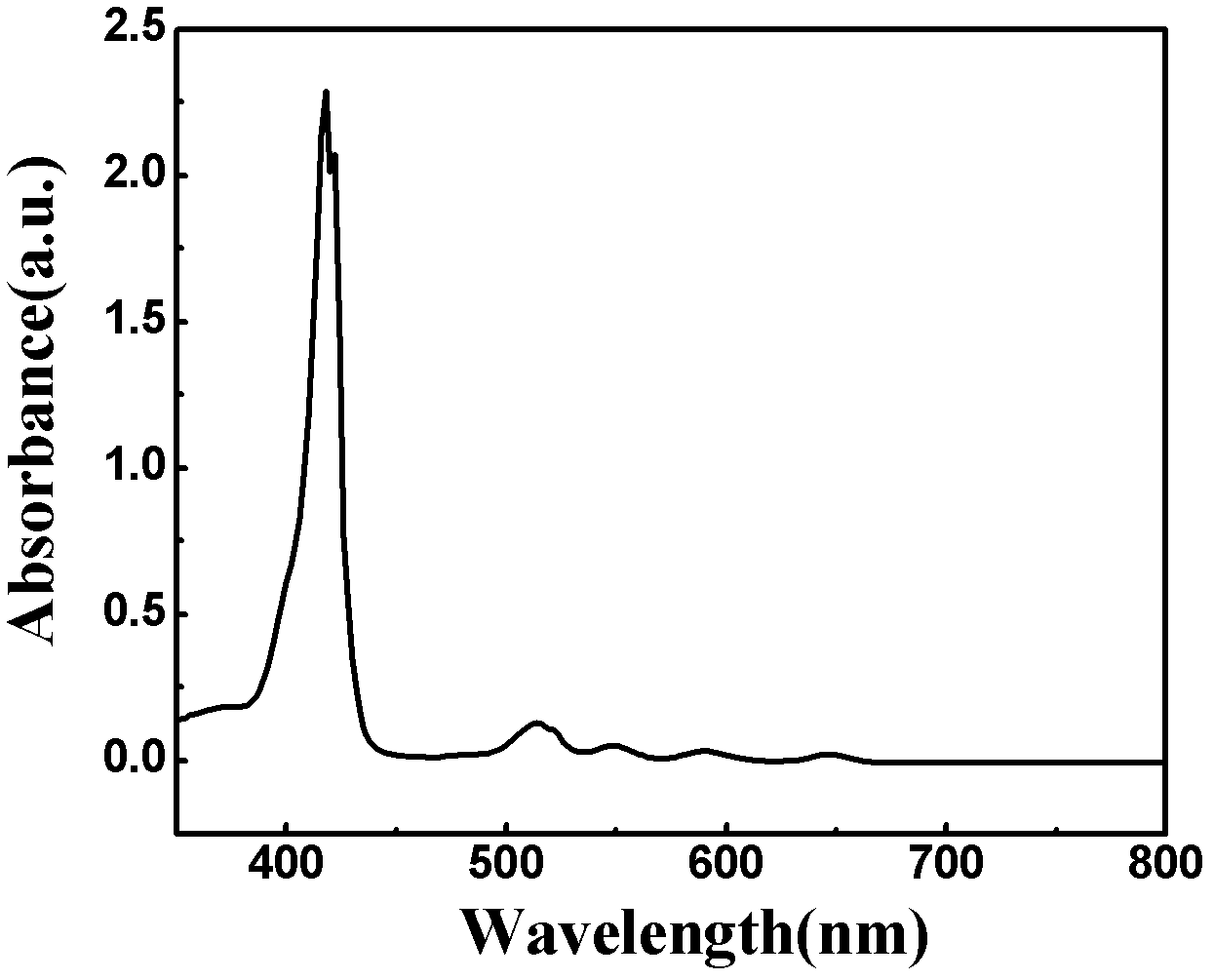
[0024] What this example prepared was the dye molecule described in the general formula, wherein X=4, M=2H, and the reaction formula is as follows:
[0025]
[0026] Firstly, p-bromobenzaldehyde (14.8g, 80mmol) was dissolved in 200ml of propionic acid, the solution was heated to reflux at 140°C, pyrrole (5.36g, 80mmol) was added dropwise, stirred for 30min, cooled to room temperature, and half of the solvent was evaporated , and add 100ml methanol, the mixture is placed in the refrigerator overnight, after filtration, use petroleum ether / dichloromethane=2 / 3 column chromatography, pass through CHCI 3 Recrystallized with methanol=1:5 to obtain purple solid compound 4-4-bromobenzoporphyrin (4.2 g, 22.6%).
[0027] 4-4-Bromobenzoporphyrin (931mg, 1mmol), bistetraalkylamine (1.86g, 4mmol), K 2 CO 3 (828mg, 6mmol) and copper powder (320mg, 5mmol) were added in anhydrous DMF (100mL), then it was 2 Heated to reflux for 24h under protection, removed DMF, and used CHCI for solid ...
Embodiment 2
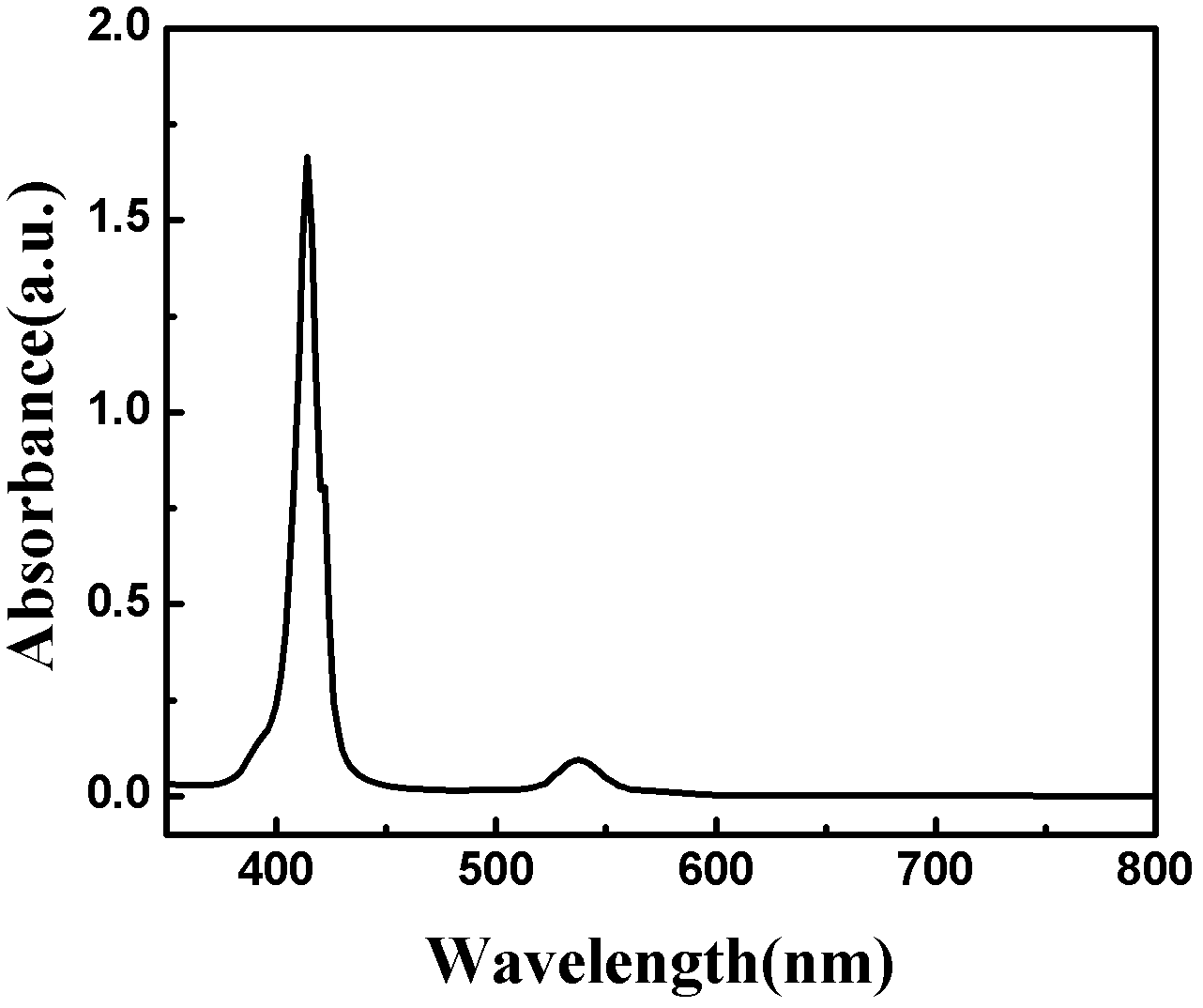
[0033] What this example prepared was the dye molecule described in the general formula, wherein X=8, M=2H, and the reaction formula is as follows:
[0034]
[0035]Firstly, p-bromobenzaldehyde (14.8g, 80mmol) was dissolved in 200ml of propionic acid, the solution was heated to reflux at 140°C, pyrrole (5.36g, 80mmol) was added dropwise, stirred for 30min, cooled to room temperature, and half of the solvent was evaporated , and add 100ml methanol, the mixture is placed in the refrigerator overnight, after filtration, use petroleum ether / dichloromethane=2 / 3 column chromatography, pass through CHCI 3 Recrystallized with methanol=1:5 to obtain purple solid compound 4-4-bromobenzoporphyrin (4.6 g, 22.9%).
[0036] 4-4-Bromobenzoporphyrin (931mg, 1mmol), bis-octaylamine (1.86g, 3mmol), K 2 CO 3 (828mg, 6mmol) and copper powder (320mg, 5mmol) were added in anhydrous DMF (100mL), then it was 2 Heated to reflux for 24h under protection, removed DMF, and used CHCI for solid 3 Ex...
Embodiment 3
[0039] What this example prepared was the dye molecule described in the general formula, wherein X=4, M=Zn, and the reaction formula is as follows:
[0040]
[0041] Firstly, p-bromobenzaldehyde (14.8g, 80mmol) was dissolved in 200ml of propionic acid, the solution was heated to reflux at 140°C, pyrrole (5.36g, 80mmol) was added dropwise, stirred for 30min, cooled to room temperature, and half of the solvent was evaporated , and add 100ml methanol, the mixture is placed in the refrigerator overnight, after filtration, use petroleum ether / dichloromethane=2 / 3 column chromatography, pass through CHCI 3 Recrystallized with methanol=1:5 to obtain purple solid compound 4-4-bromobenzoporphyrin (3.9 g, 21.6%).
[0042] 4-4-Bromobenzoporphyrin (931mg, 1mmol), bistetraalkylamine (1.86g, 4mmol), K 2 CO 3 (828mg, 6mmol) and copper powder (320mg, 5mmol) were added in anhydrous DMF (100mL), then it was 2 Heated to reflux for 24h under protection, removed DMF, and used CHCI for solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com