Pet radiotracers for imaging fatty acid metablolism and storage
A technology of fatty acids and radioisotopes, applied in the direction of in vivo radioactive preparations, preparation of X-ray contrast agents, preparations for in vivo experiments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Example 1: Imaging of small animals.
[0212] Animal preparation. All animal procedures were performed in compliance with the guidelines for the care and use of animals established by the Washington University Animal Research Committee. Animal preparation was performed as previously described. 24-26 . Mice were placed in metabolic cages and anesthetized by inhalation of 2%-2.5% isoflurane in an induction chamber. Anesthesia was maintained throughout imaging with 1%-1.5% isoflurane delivered through a custom-made nose cone. Venous access is via the exopharyngeal vein. Body temperature is maintained with circulating water blankets and heat lamps. Monitor heart and respiration rates throughout the procedure.
[0213] PET collection. Animals were secured in custom-made acrylic restraints and placed within the field of view of the small animal imaging PET scanner. Imaging acquisition was started 5 s after the tracer was injected via the right jugular catheter. Imagi...
Embodiment 2
[0215] Example 2: Large animal imaging.
[0216] Animal preparation. Purpose-bred ~6-10 kg male Beagle dogs were fasted, anesthetized and instrumented as previously described 3,4 . One femoral vein is catheterized for dosing. For arterial samples, a catheter was placed in the thoracic aorta through the femoral artery and arterial blood pressure was monitored. To obtain a venous blood pressure sample, a coronary sinus cannula can be placed through the right external jugular vein under fluoroscopic guidance as previously described 28 . ECG, arterial blood pressure and heart rate can be detected throughout the process. All measurements can be performed on the microPET Focus 220. All procedures were performed in accordance with the guidelines for the care and use of research animals.
[0217] PET imaging protocol. Two imaging schemes can be used.
[0218] plan 1. Transmission scans were initially used to correct for positron decay. After a transmission scan, perform a 5...
Embodiment 3
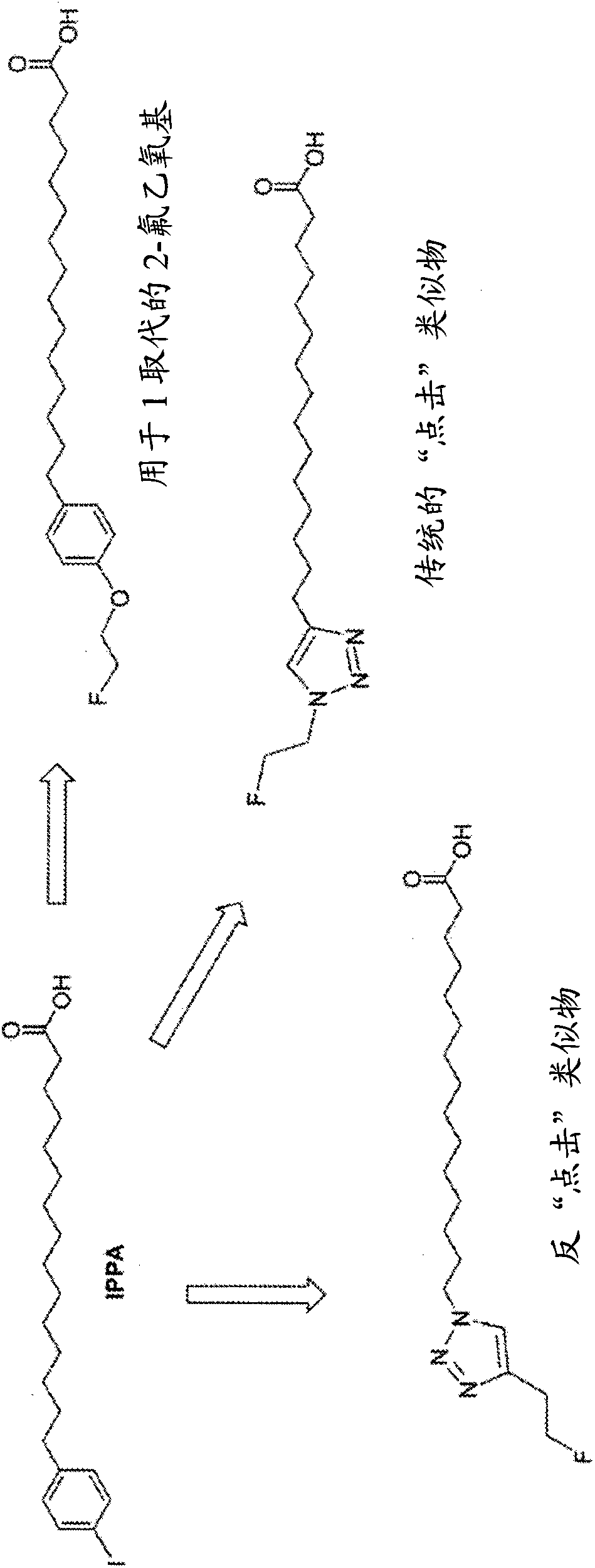
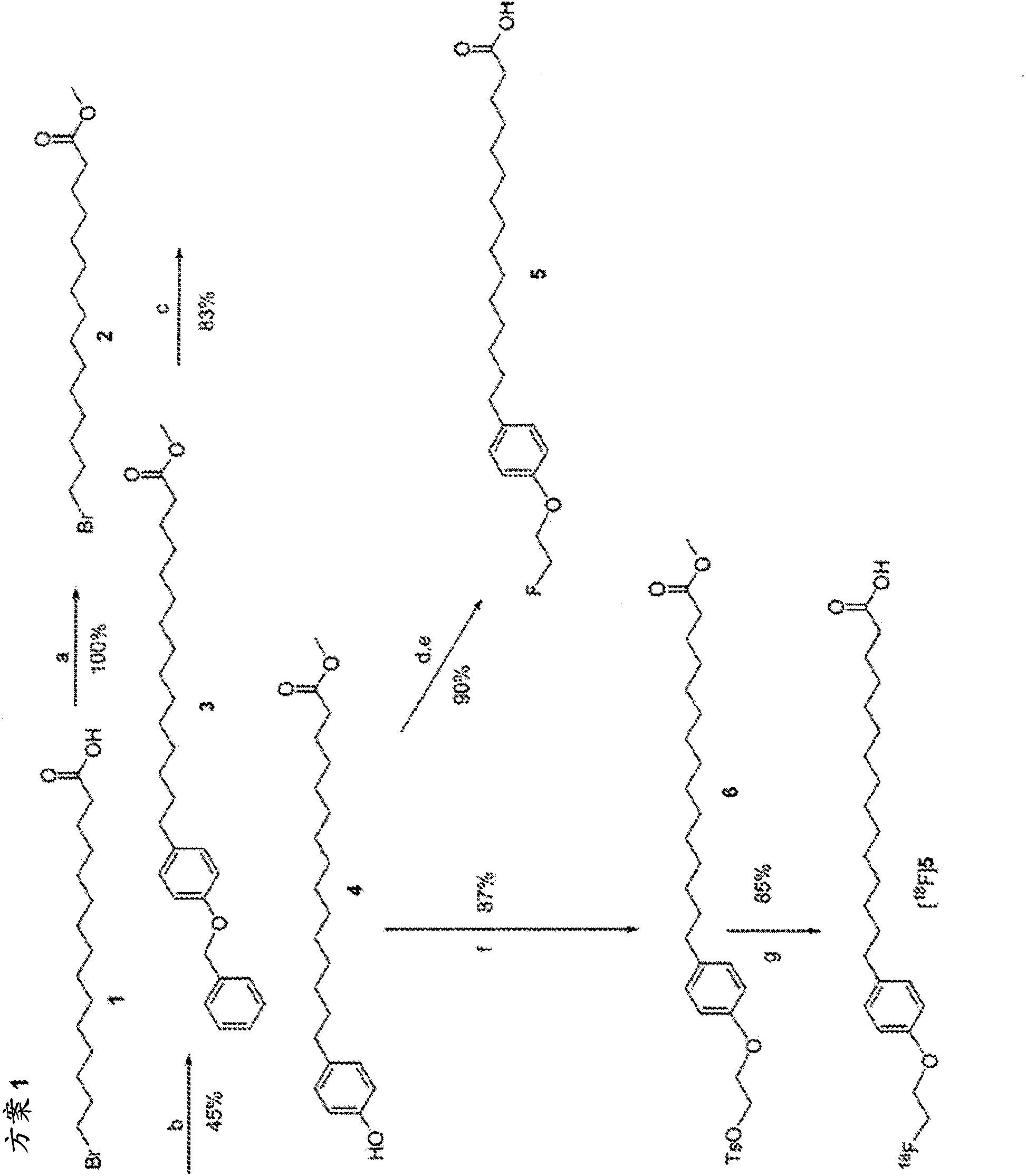
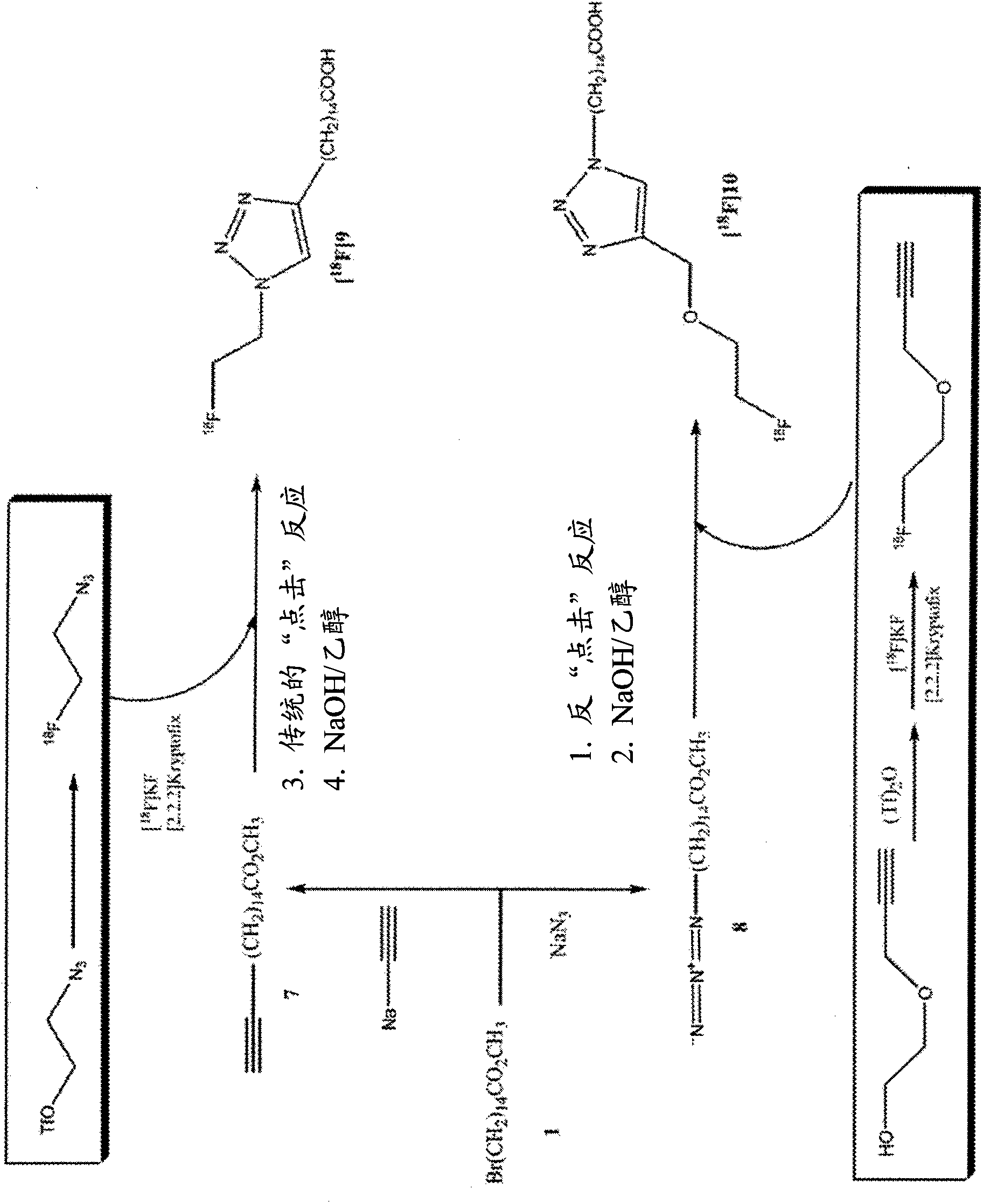
[0221] This example shows the flexibility of our strategy, initially comparing the 60 min kinetics of the 2-fluoroethoxy analogue for IPPA to that in the same animal ( Figure 4 )of 11 C-palmitate ( Figure 4 ) kinetic comparison. Imaging for both radiotracers was 60 min. use 11 C-palmitate (top) and with 18 Composite myocardial microPET images of fed mice studied with F-FAA-labeled novel fatty acid analogs (bottom). Images are shown from the horizontal axis and present data obtained 20-30 min after tracer injection. 18 F-FAA Image Ratio 11 The C-palmitate image shows excellent quality and high tracer activity. Figure 4 Individual microPET images are presented. The top rows (18-13330 and 19-12011) are 11 C-palmitate image and the top rows (18-22135 and 19-21952) are 18 F-FAA image. Increasing signal strength is indicated by green to yellow to red (highest). Relatively similar tracer kinetics were noted ( Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com