Thermo sensitive in situ gel loaded with drug by chemical bonds, and preparation method thereof
An in-situ gel, drug-loading technology, applied in the field of drug-containing polymer gels, can solve the problems of difficult industrialization, harsh preparation conditions, and high production costs, achieve slow and long-lasting drug release, increase drug loading, and prepare simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
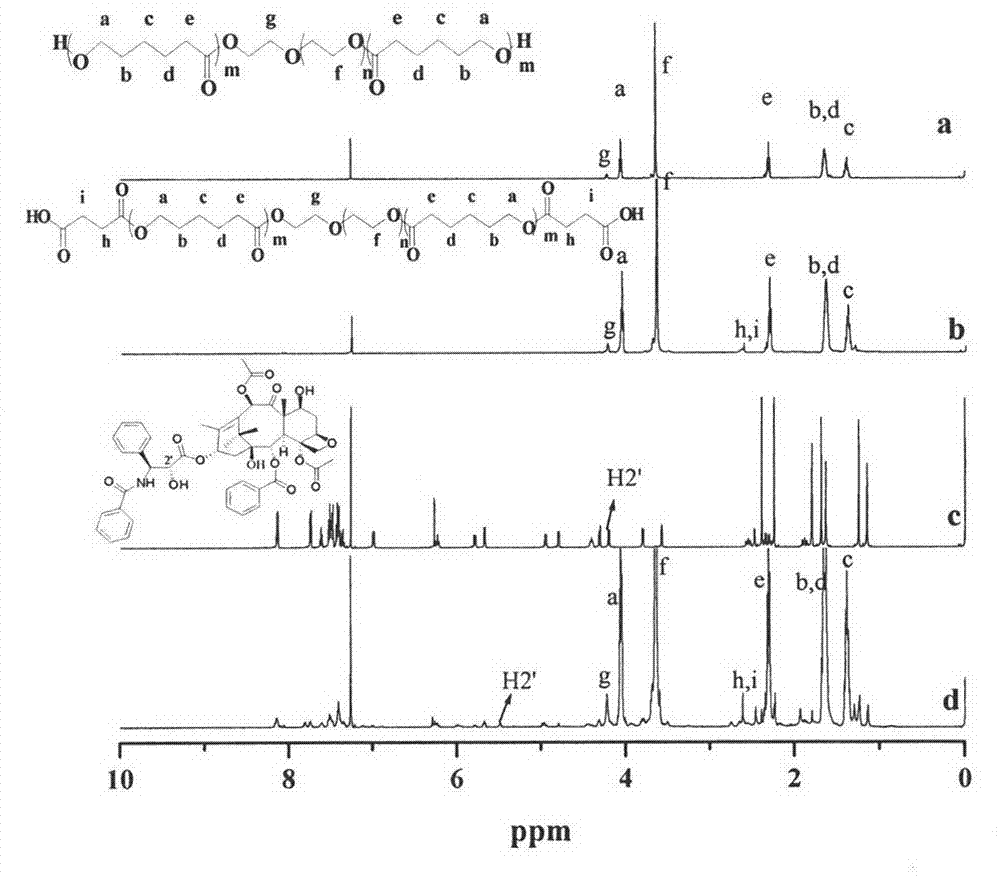
[0026] Polymer and drug ester bond connection:
[0027] Add 1.5g dihydroxy PEG (number average molecular weight 1500Da), 2mLε-caprolactone, 117μl stannous octoate (Sn(Oct) 2 ), evacuated and filled with nitrogen. The reaction was terminated after 6h at 140°C. Dissolve with dichloromethane, precipitate with ether, and obtain polycaprolactone (PCL)-PEG-PCL by vacuum filtration. Put 3.5 g of PCL-PEG-PCL and 0.22 g of succinic anhydride into a dry flask, and evacuate nitrogen. The reactant was reacted at 140° C. for 5 hours, then cooled to room temperature, dissolved in dichloromethane, precipitated with ether, and vacuum filtered to obtain COOH-PCL-PEG-PCL-COOH. Then add 0.35g COOH-PCL-PEG-PCL-COOH, 0.17g paclitaxel (PTX) and 82.52mg dicyclohexylcarbodiimide (DCC) into the dry reaction tube, and then dissolve dimethylformamide (DMF). 48.87 mg of 4-dimethylaminopyridine (DMAP) was added. Vacuum and fill with nitrogen. React at room temperature for 24h. The reaction solution was...
Embodiment 2- Embodiment 13
[0029] According to the method of Example 1, the composition and type of polyethylene glycol, polyester, and drugs were changed to obtain a variety of PEG-polyesters (I-2 to I-13) with different C / BAB / C type bonded drugs and their The specific parameters of the temperature-sensitive in-situ gel are shown in Table 1.
[0030] Table 1 C / B-A-B / C type (ester bond) drug-bonded PEG-polyester block copolymer and its temperature-sensitive in-situ gel
[0031]
[0032]
[0033] M PEG :The number average relative molecular mass of PEG; M PE : Number average relative molecular mass of polyester; N: molar ratio of drug to polyester block; W ABC : Mass ratio of hydrophobic block / hydrophilic block (hydrophobic drug content is included in the mass of the hydrophobic block, and hydrophilic drug content is included in the mass of the hydrophilic block); C gel :The PEG-polyester content of the bonded drug in the in-situ gel system; T gel : Gelation temperature region; PTX: paclitaxel; docetaxe...
Embodiment 14
[0035] Example 14 (II-1)
[0036] Add 1.0g polyethylene glycol monomethyl ether (mPEG) (number average molecular weight 750Da), 3.2mLε-caprolactone, 100μl stannous octoate (Sn(Oct) 2 ), evacuated and filled with nitrogen. The reaction was terminated after 6h at 140°C. It was dissolved in dichloromethane, precipitated with ether, and filtered under vacuum to obtain mPEG-PCL. Put 3.15g mPEG-PCL and 0.11g succinic anhydride into a dry flask, evacuated and ventilated nitrogen. The reactants were reacted at 140°C for 5 hours, then cooled to room temperature, dissolved in dichloromethane, precipitated with ether, and vacuum filtered to obtain mPEG-PCL-COOH. Then add 0.32g mPEG-PCL-COOH, 0.17g paclitaxel (PTX) and 82.52mg dicyclohexylcarbodiimide (DCC), dimethylformamide (DMF) to dissolve in the dry reaction tube, and finally add 48.87mg4 -Dimethylaminopyridine (DMAP). Vacuum and fill with nitrogen. React at room temperature for 24h. The reaction solution was dialyzed in DMF for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com