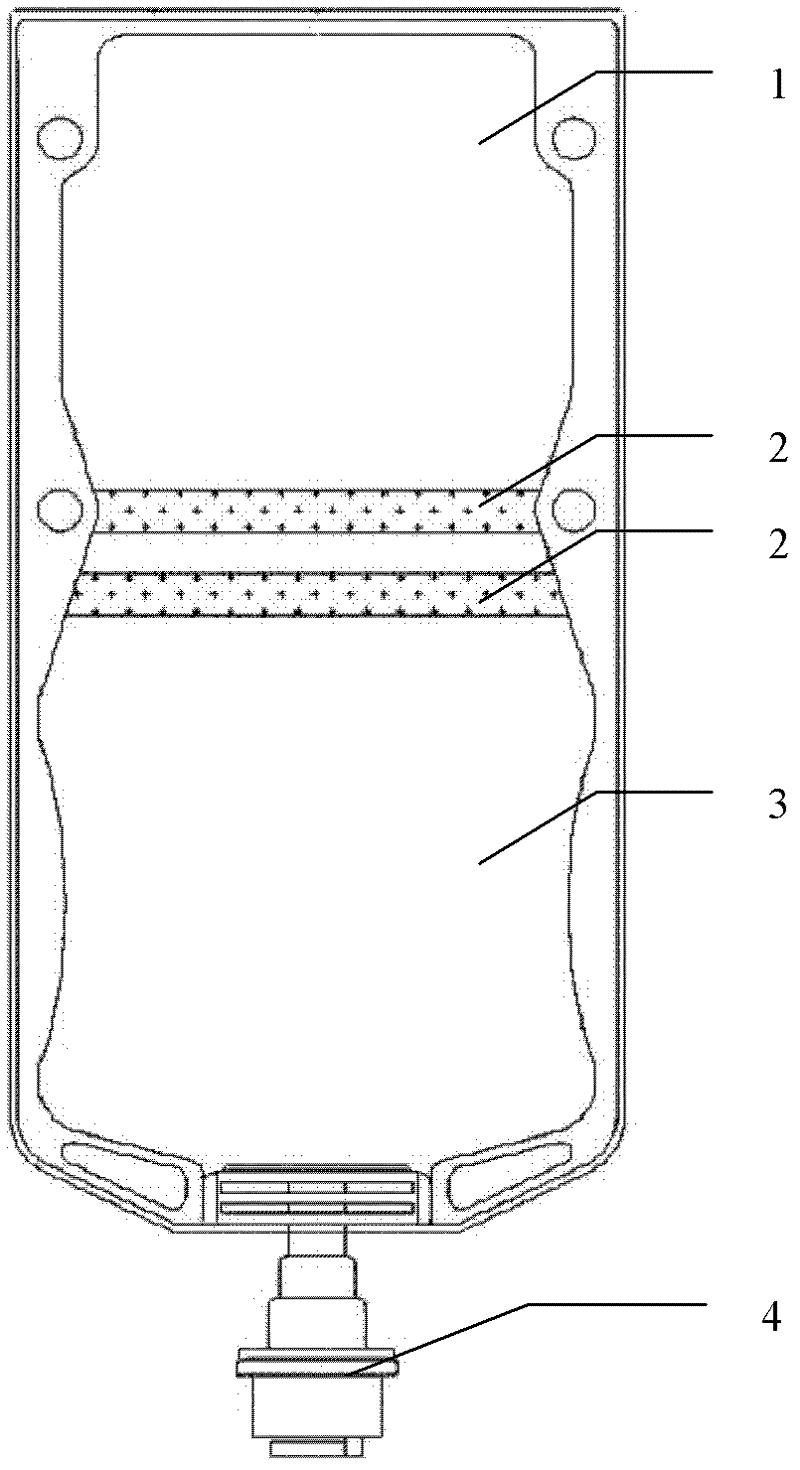
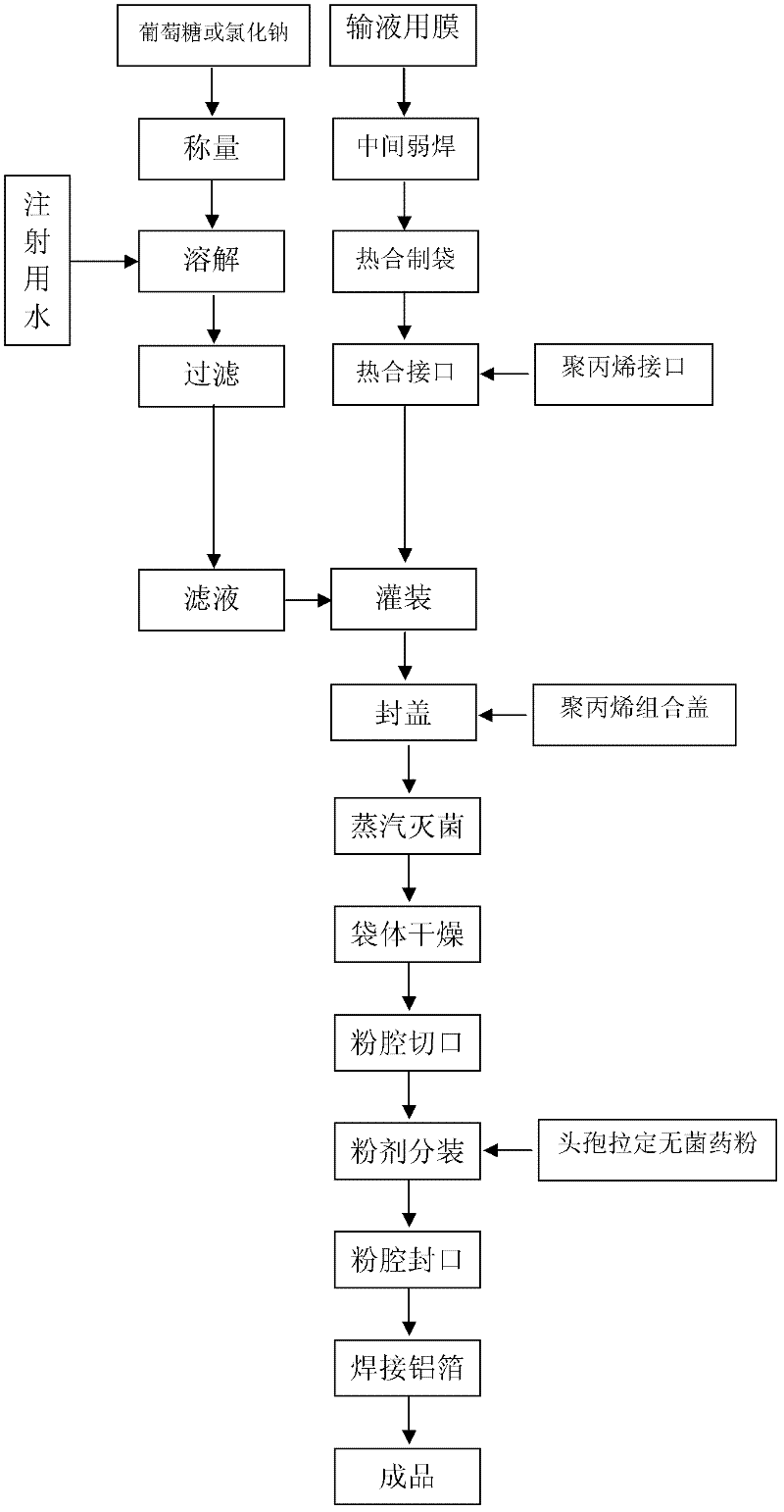
Cefradine injection packed by double-cavity bag and preparation method thereof
A technology of cephradine and injection, which is applied in the field of pharmaceutical composition and its preparation, can solve problems such as inability to airdrop, affect product safety, and time-consuming process of dissolving and diluting, so as to improve effectiveness and safety and prolong product validity period , enhance the effect of environmental adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0038] The samples prepared in Example 1 were put into glass infusion bottles and double-chamber bags respectively, and the stability investigation was carried out after sterilization. It can be found that the samples were investigated for 6 months in the accelerated test at 40°C and in the long-term test at 25°C for 12 months after sterilization. After one month, each index of the sample of double-chamber bag packaging has no significant change, and the stability is obviously better than that of glass infusion bottle packaging samples. (As shown in Table 1)
[0039] Table 1 Product Stability Investigation Comparison Results
[0040]
[0041]
experiment example 2
[0043] Get 10 cephradine powder injections (in cillin bottle, specification: 0.5g) and 10 bottles of glucose injection (in glass infusion bottle, specification: 100ml: 0.5g) respectively, and transfer cephradine sterile medicine powder into glucose Injection, and make it dissolve completely; Another get 10 bags adopting the double-chamber bag sample that embodiment 1 prepares, open weak welding rod, shake and make cephradine sterile powder dissolve completely, and the operation process is carried out comparative investigation. (As shown in Table 2, the test data is the average value of 10 tests.)
[0044] Table 2
[0045]
[0046] As can be seen from the comparison results, the use of the existing common packaged cefradine preparations is relatively complicated to operate and has high environmental requirements (currently large hospitals generally require operations to be performed on clean operating benches with laminar flow protection), and insoluble particles are likely ...
experiment example 3
[0048] Get respectively 50 cephradine powder injections (penillin bottled, specification: 0.5g) of two manufacturers and 50 bags of double-chamber bag samples prepared in Example 1, and place them under the accelerated test conditions at 40°C to investigate June. Samples all adopt the mode that puts upside down, take out 10 (bags) respectively in accelerating 0, 1, 2, 3, 6 months, cephradine sterile medicine powder is mixed with the solubility of 0.1g / ml with purified water, observe the clarification of solution degree, the results are shown in Table 3:
[0049] table 3
[0050]
[0051] From the investigation data in the above table, it can be known that the product packaged in double-chamber bags has significantly better clarification results of cephradine sterile powder than the products packaged in vials currently on the market, so products packed in double-chamber bags are safer.
[0052] Analyzing the reason, it may be that the composition of the rubber stopper is mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com