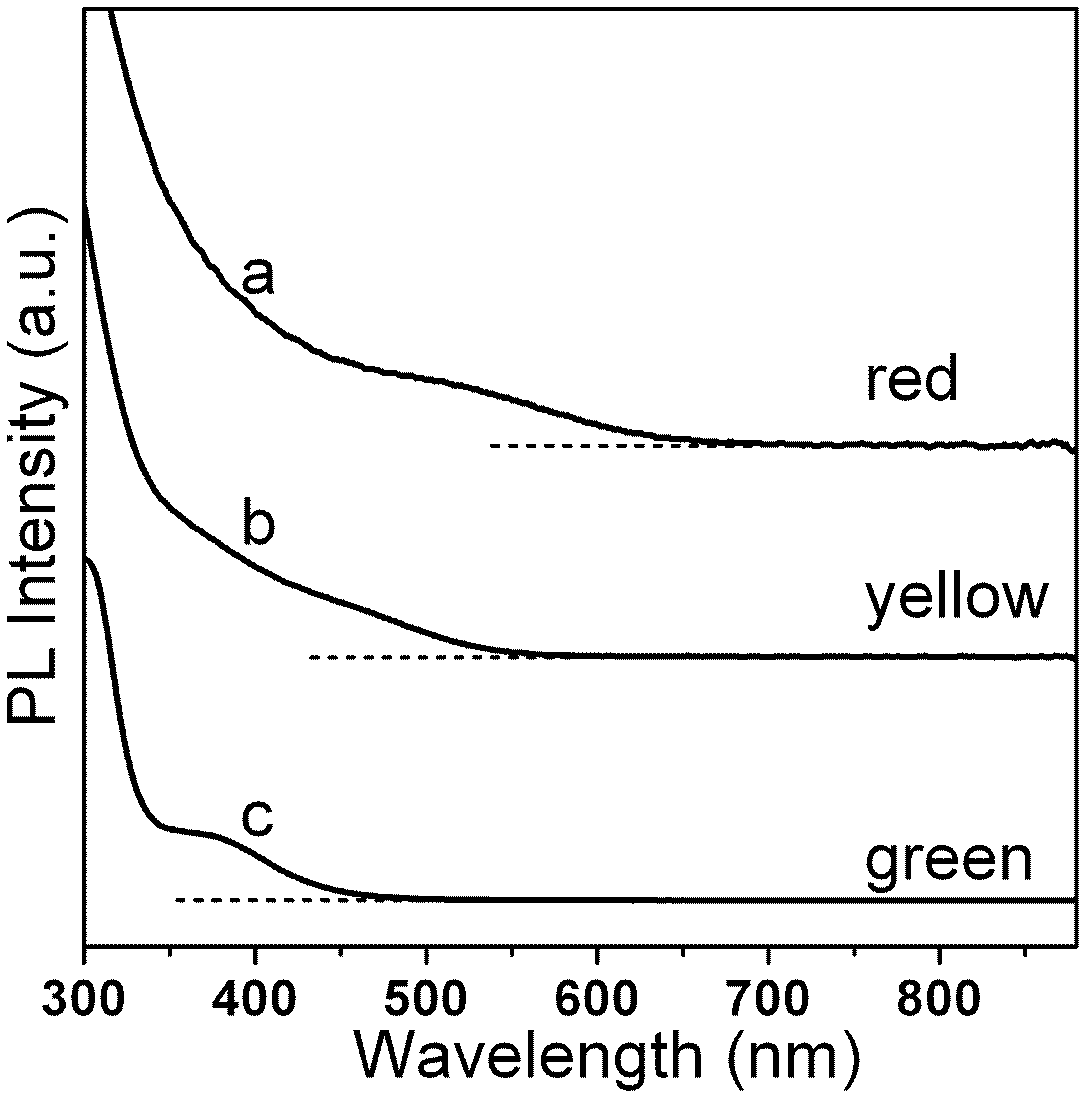
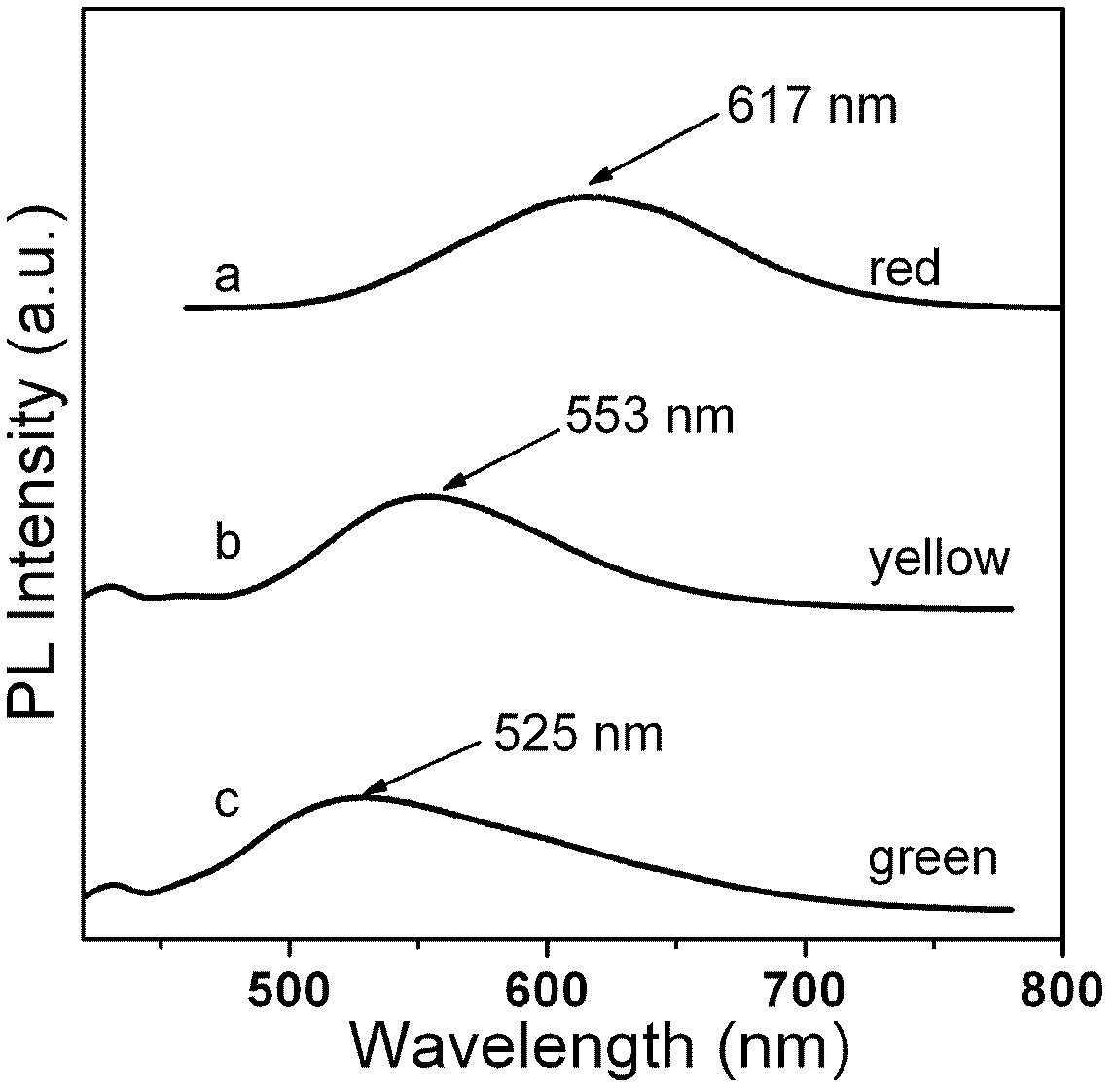
Nano-crystal fluorescent powder and preparation method thereof
A technology of nanocrystal and phosphor, which is applied in the field of nanocrystal phosphor and its preparation, and core-shell nanocrystal phosphor, which can solve the problems of long time-consuming, complicated operation process, difficulty in large-scale production, etc., and achieve high yield , Large light absorption coefficient and narrow band gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Step 1. Preparation of reaction source
[0055] Add 0.19g of cuprous iodide, 1.16g of indium acetate, 5mL of dodecyl mercaptan and 25mL of octadecene into a 100mL three-neck flask and mix to obtain a mixed solution 1. Heat the mixed solution 1 to 120°C in vacuum and stir for 30min Then add 2.5mL oleic acid, continue to stir at 120°C in vacuum for 30min until it is completely dissolved, then pass in nitrogen gas for 30min, then raise the temperature to 220°C, the solution turns from light yellow to dark red, and react at constant temperature for 1h to obtain the reaction source.
[0056] Step 2, preparation of colloidal solution
[0057] Mix 2.64g of zinc acetate, 10mL of oleylamine and 10mL of octadecene to obtain a cloudy mixed solution 2, heat the mixed solution 2 to 50°C in vacuum and stir for 30 minutes, then pass nitrogen gas for 30 minutes and raise the temperature to 120°C until The turbid mixed solution 2 becomes clear, and the zinc source is prepared; at 220°C...
Embodiment 2
[0063] Step 1. Preparation of reaction source
[0064] Add 0.19g of cuprous iodide, 1.16g of indium acetate, 5mL of dodecyl mercaptan and 25mL of octadecene into a 100mL three-necked flask and mix to obtain a mixed solution 1, and heat the mixed solution 1 to 100°C in vacuum and stir for 40min ; Then add 2.5mL oleic acid, continue to stir at 120°C for 30min until completely dissolved, then pass through nitrogen for 30min, then raise the temperature to 200°C, the solution turns from light yellow to deep red, and react at constant temperature for 1h to obtain the reaction source.
[0065] Step 2, preparation of colloidal solution
[0066] Mix 2.64g of zinc acetate, 10mL of oleylamine and 10mL of octadecene to obtain a cloudy mixed solution 2, heat the mixed solution 2 to 80°C in vacuum and stir for 30 minutes, then pass nitrogen gas for 30 minutes, and then raise the temperature to 140°C, The zinc source is prepared until the turbid mixed solution 2 becomes clear; the zinc sour...
Embodiment 3
[0072] Step 1. Preparation of reaction source
[0073] Add 0.19g of cuprous iodide, 1.16g of indium acetate, 5mL of dodecyl mercaptan and 25mL of octadecene into a 100mL three-neck flask and mix to obtain a mixed solution 1. Heat the mixed solution 1 to 110°C in vacuum and stir for 60min Then add 2.5mL oleic acid, continue to stir at 120°C for 30min until completely dissolved, then pass nitrogen gas for 30min, then raise the temperature to 230°C, the solution turns from light yellow to deep red, and react at constant temperature for 1h to obtain the reaction source.
[0074] Step 2, preparation of colloidal solution
[0075] Mix 2.64g of zinc acetate, 10mL of oleylamine and 10mL of octadecene to obtain a cloudy mixed solution 2, heat the mixed solution 2 to 100°C in vacuum and stir for 30 minutes, then pass in argon for 30 minutes, and then raise the temperature to 160°C , until the turbid mixed solution 2 becomes clear, the zinc source is prepared; the zinc source is added d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com