Application of arctigenin in preparation of medicines for preventing and treating bone marrow suppression caused by radiation or chemicals
A technology of arctigenin and bone marrow suppression, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem that there are no literature reports on arctigenin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
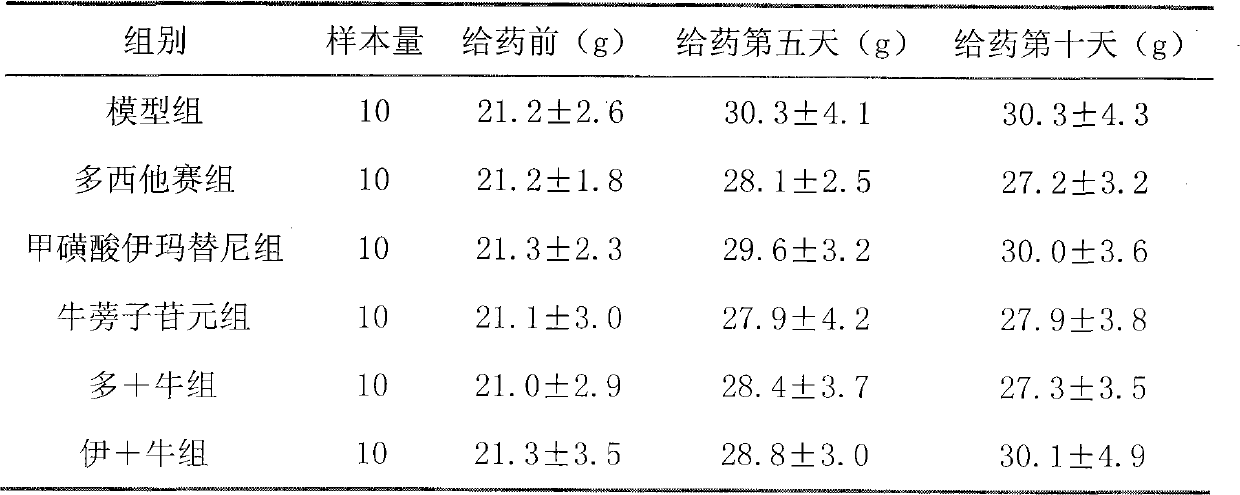
[0020] Example 1 Effect of arctigenin on bone marrow suppression induced by chemotherapy in tumor mice
[0021] 1. Animals:
[0022] Kunming mice, male and female, 7 weeks old, 18-22g, tested at (20±1)°C, humidity 40%-70%, free to drink water, and fed normally.
[0023] 2. Method:
[0024] Mouse ascites tumor s180 cells were cultured in 1640 medium, 37°C, 5% CO 2 Under conventional culture, subculture once every two days on average, and prepare a density of 3.0×10 with normal saline in the logarithmic growth phase. 7cells / ml single cell suspension, injected into the peritoneal cavity of mice under aseptic conditions, the abdominal cavity of the mice was obviously swollen 7 days after inoculation, at this time, they were killed by neck dislocation, and soaked in a beaker filled with 75% ethanol After 2-3 minutes, put the sterilized mouse into the ultra-clean workbench, expose the abdomen, extract the ascites with a sterile syringe and put it into a sterile reagent bottle for...
Embodiment 2
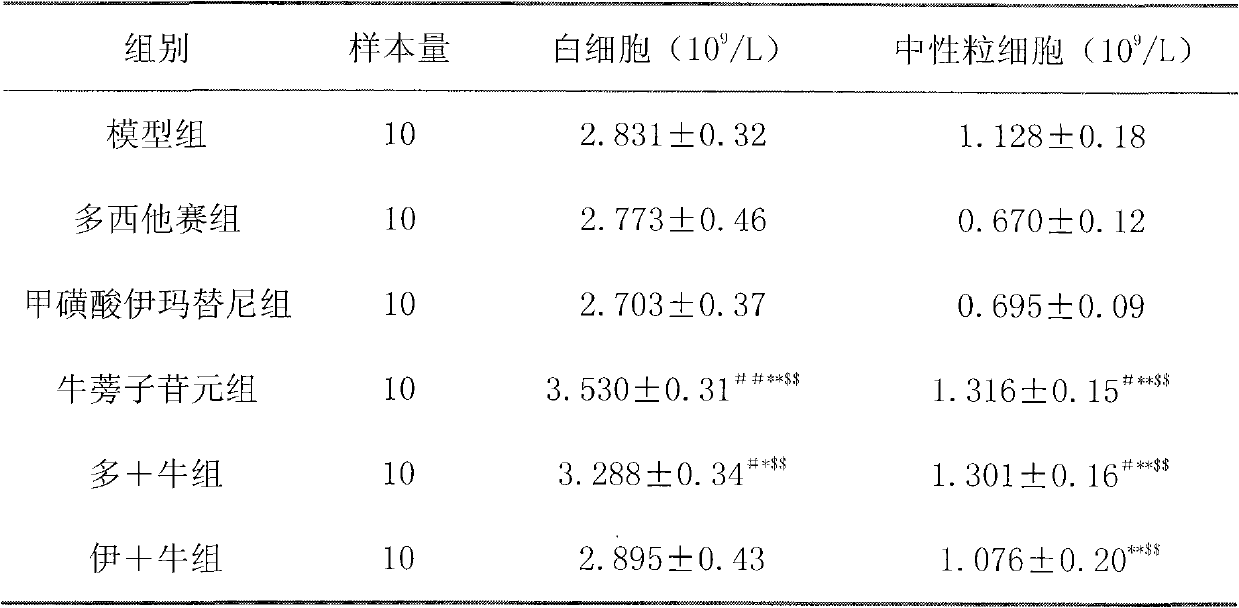
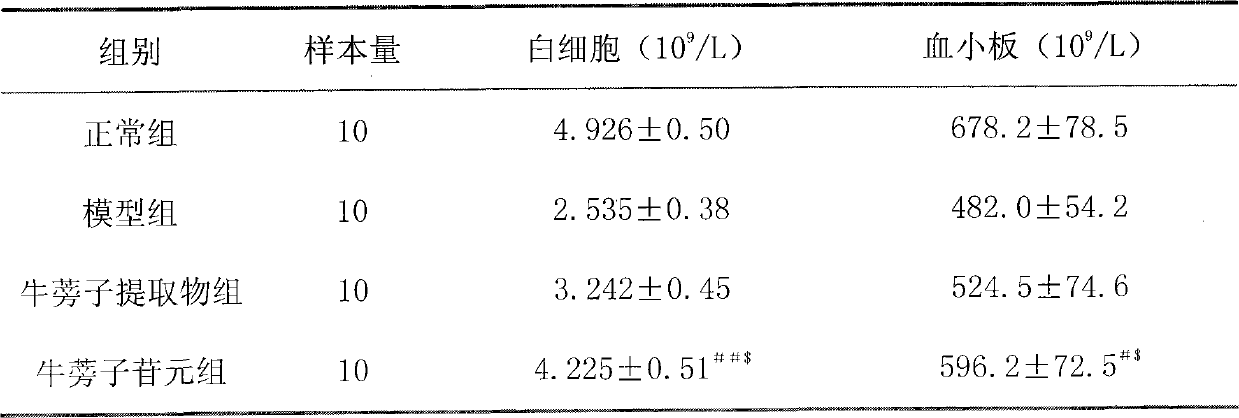
[0044] Example 2 arctigenin pair 60 Effect of Co-irradiated mice on blood cell count
[0045] 1. Animals:
[0046] Kunming mice, male and female, 7 weeks old, 18-22g, tested at (20±1)°C, humidity 40%-70%, free to drink water, and fed normally.
[0047] 2. Method:
[0048] In addition to the normal group, mice were irradiated with 4Gy 60 Co radiation, using a one-time irradiation. On the 3rd, 7th, and 10th day, blood was drawn from the orbital vein to detect the complete blood cell count.
[0049] The irradiated mice were randomly divided into the following model group, burdock fruit extract group and arctigenin group, with 10 mice in each group, half male and half male. Each group was treated or administered as follows.
[0050] The first group is the normal group: intraperitoneal injection of 0.9% normal saline;
[0051] The second group is the model group: intraperitoneal injection of 0.9% normal saline;
[0052] The third group is the burdock fruit extract group: 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com