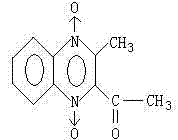
Method for synthesizing mequindox
A technology for the synthesis of acemetquine and its synthesis method, which is applied in the field of organic chemical synthesis, can solve the problems of unreported improvement of the synthesis process of acemetquine, great influence on the yield, difficulty in control, etc., and achieve easy control, improved catalytic efficiency, and side effects Response reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthetic method of methaquine, comprises the steps:
[0027] (1) Preparation of benzoxadiazepine-N-oxide (ie BFO):
[0028] In a 1000mL three-necked flask, add 360g of liquid caustic soda (concentration: 30%) and 480g of water, cool it with water to about 25°C, start aerating chlorine gas, aerate and weigh until 98g is completed, and obtain a sodium hypochlorite solution, which is all added to a 2000mL three-necked flask , stir and add 120g of liquid caustic soda, cool down to 30°C, add 130g of o-nitroaniline, control the reaction temperature at 45-50°C, keep it at this temperature for 2 hours, then cool to below 30°C, and obtain BFO yellow crystals by suction filtration The powder is 137g, and the water content is about 6.5%.
[0029] (2) Preparation of acemethaquine:
Embodiment 2
[0033] A kind of synthetic method of methaquine, comprises the steps:
[0034] Other operations are the same as embodiment 1, the difference is:
[0035] (2) Preparation of acemethaquine:
Embodiment 3
[0039] A kind of synthetic method of methaquine, comprises the steps:
[0040] Other operations are the same as embodiment 1, the difference is:
[0041] (2) Preparation of acemethaquine:
[0042] Add the mother liquor in step (2) of Example 2 and 48g of BFO (4% water) into a 250mL three-necked flask, heat to dissolve and clarify, the temperature rises to 60°C, then add 34.8g of acetylacetone, and then fractionate at 60-65°C Add 2.8g of anhydrous sodium acetate catalyst at a time until the solid is released, all the catalyst is added, and then keep warm at about 65°C for 3 hours, then cool to 30°C and filter with suction to obtain 68g of acemethaquine solid product.
[0043] This product is a bright yellow crystalline powder. According to the 1992 edition of the "Chinese Veterinary Drug Standards", the melting point of the dry product of acetylmethaquine is 155.2°C. The infrared absorption spectrum of this product should be consistent with the control spectrum. The conten...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com