Troxipide dispersible tablet and preparation method thereof
A technology for traxipide and dispersible tablets, applied in the field of medicine, can solve the problems of long disintegration time and single taking method, and achieve the effects of short disintegration time, high bioavailability and good dispersion uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1. The selection of disintegrating agent
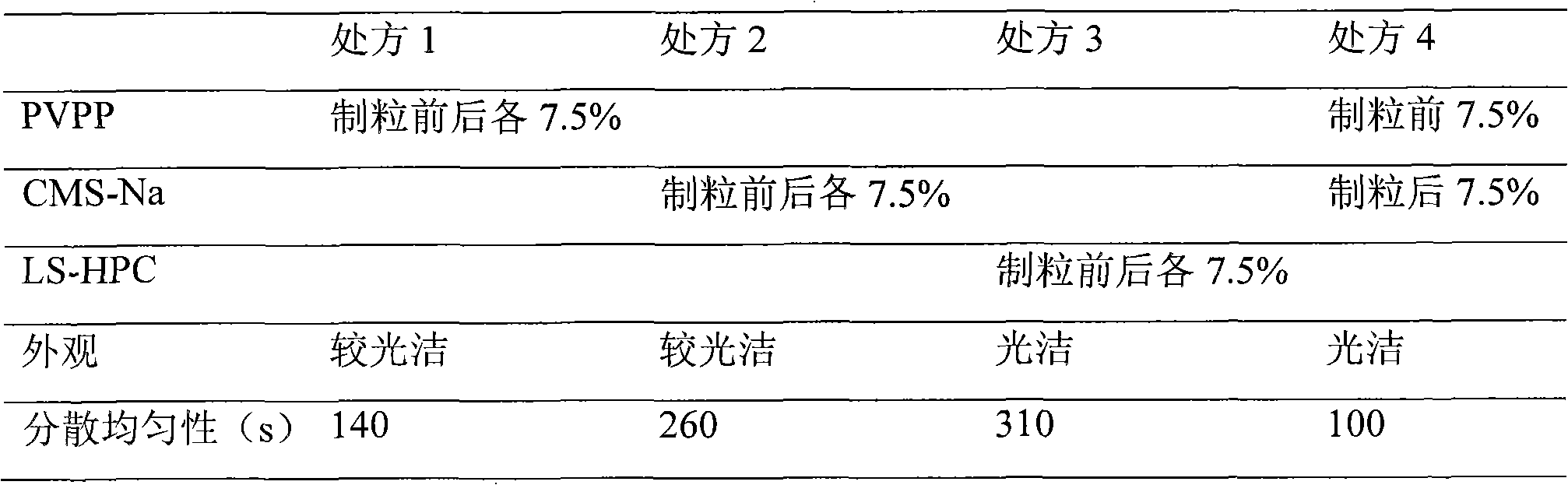
[0017] Select PVPP (cross-linked polyvinylpyrrolidone), CMS-Na (sodium carboxymethyl starch), LS-HPC (low-substituted hydroxypropyl cellulose) as disintegrants, the dosage is 15%, each before and after granulation Add half, then detect the appearance and pink uniformity of the tablet, the results are shown in Table 1.
[0018] Table 1. Preliminary selection of disintegrants
[0019]
[0020] For the four prepared tablets, the disintegrating agent LS-HPC added in prescription 3 had almost no disintegration effect; when disintegrating in prescription 2, the surface layer disintegrated faster, while the inside was more difficult to disintegrate, but the tablet The appearance of the formulation is the best; the disintegrating agent added in prescription 1 has a greater effect, but the appearance is loose and there are cracks; the appearance and dispersion uniformity of prescription 4 all meet the requirements, which ...
Embodiment 2
[0021] Example 2. Selection of Adhesive
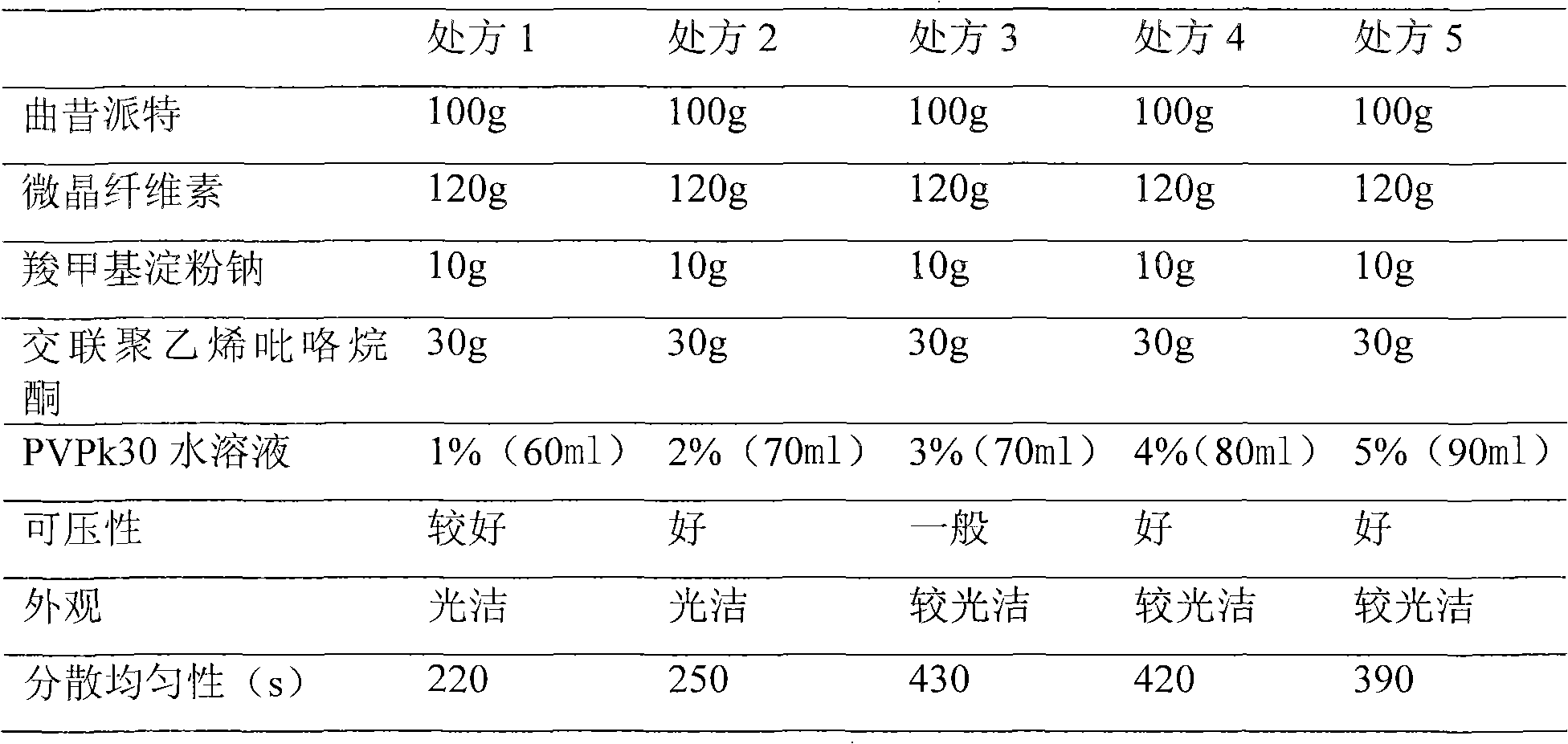
[0022] Based on the selection of filler MCC (microcrystalline cellulose) and disintegrant PVPP (cross-linked polyvinylpyrrolidone), CMS-Na (sodium carboxymethyl starch), the concentration of the binder is investigated, and the dispersion uniformity is the main Index, Tablet Appearance Compressibility as the secondary index to select the best formulation. The results are shown in Table 2.
[0023] Table 2. Choice of Binder Concentration
[0024]
[0025] Note: Measuring method for dispersion uniformity (s): Take 2 tablets of the test product, place in 100ml water at 20°C±1°C, shake, and record the total disintegration time.
[0026] It can be seen from Table 2 that considering the tablet dispersion uniformity, appearance and compressibility, the most preferred 2% PVPk30 aqueous solution is the binder.
Embodiment 3
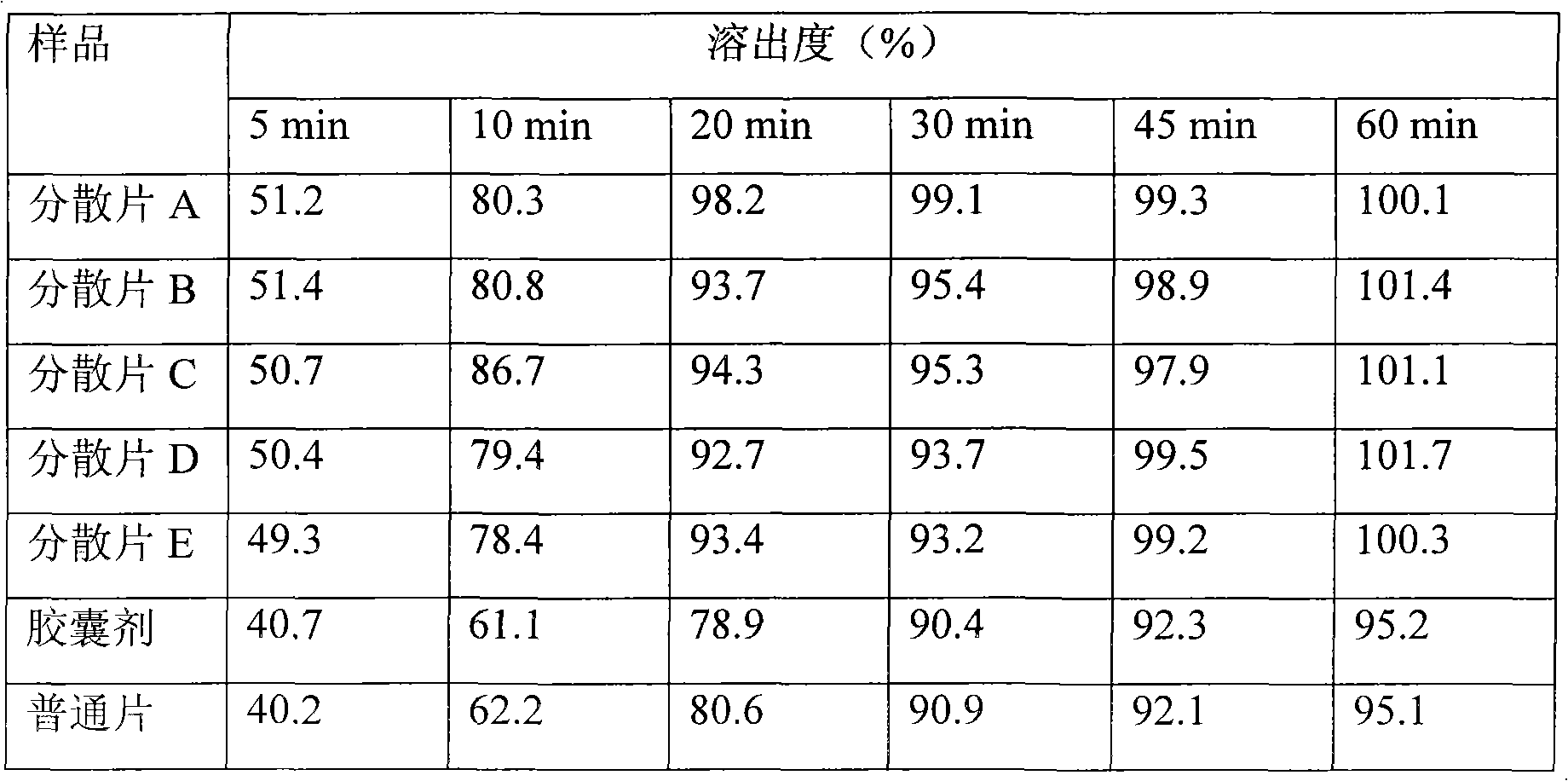
[0027] Embodiment 3. the preparation of troxipide dispersible tablet A
[0028] Prescription: Traxipide 100g
[0029] Microcrystalline Cellulose 120g
[0030] Sodium carboxymethyl starch 10g
[0031] Cross-linked polyvinylpyrrolidone 30g
[0032] Magnesium Stearate 1.3g
[0033] Micronized silica gel 1.3g
[0034] Get the prescription amount of troxipide, microcrystalline cellulose, cross-linked polyvinylpyrrolidone and sodium starch glycolate, sieve and mix evenly, use 60mL of 1% PVPk30 aqueous solution (concentration unit is g / mL, that is, 1g PVPk30 is dissolved in 100mL of water (the same below) made of soft materials, granulated with 20-mesh nylon sieve, dried at 70°C for 2 hours, granulated with 24-mesh nylon sieve, then added with micropowder silica gel and magnesium stearate, mixed evenly, measured content, pressed into tablets, after passing the inspection Package.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com