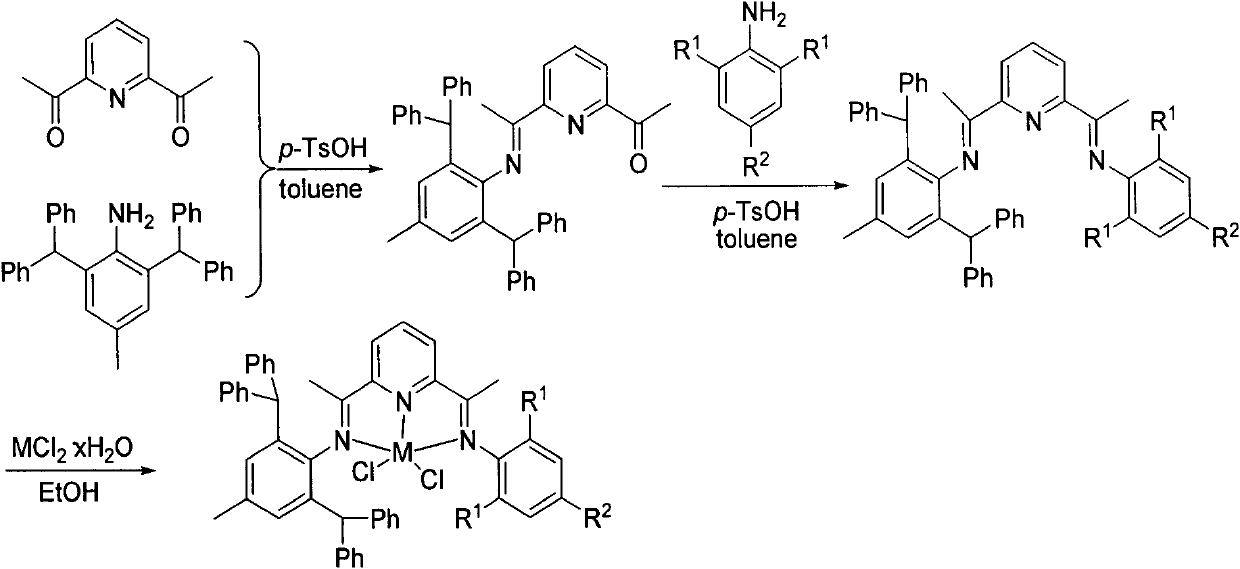
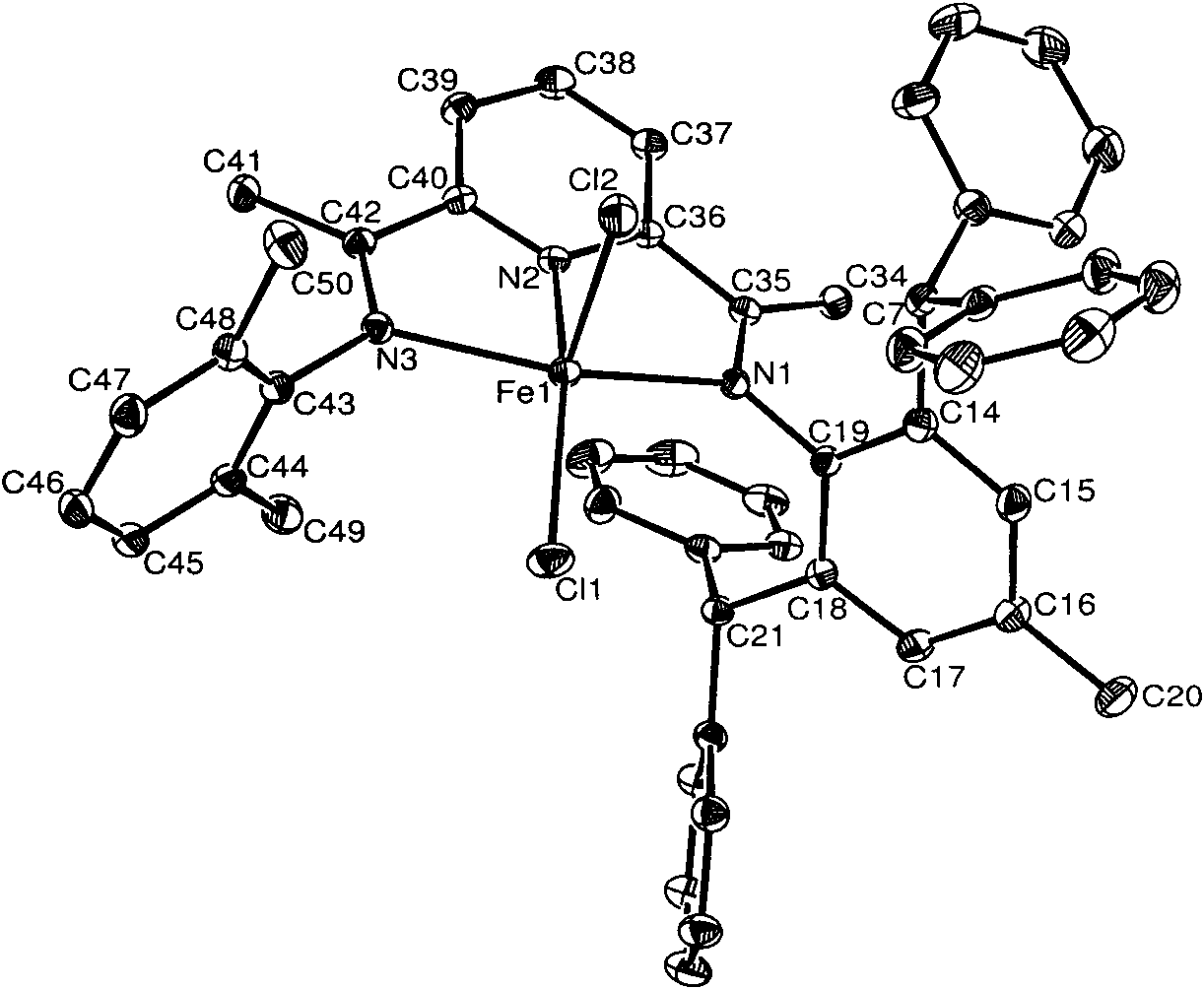
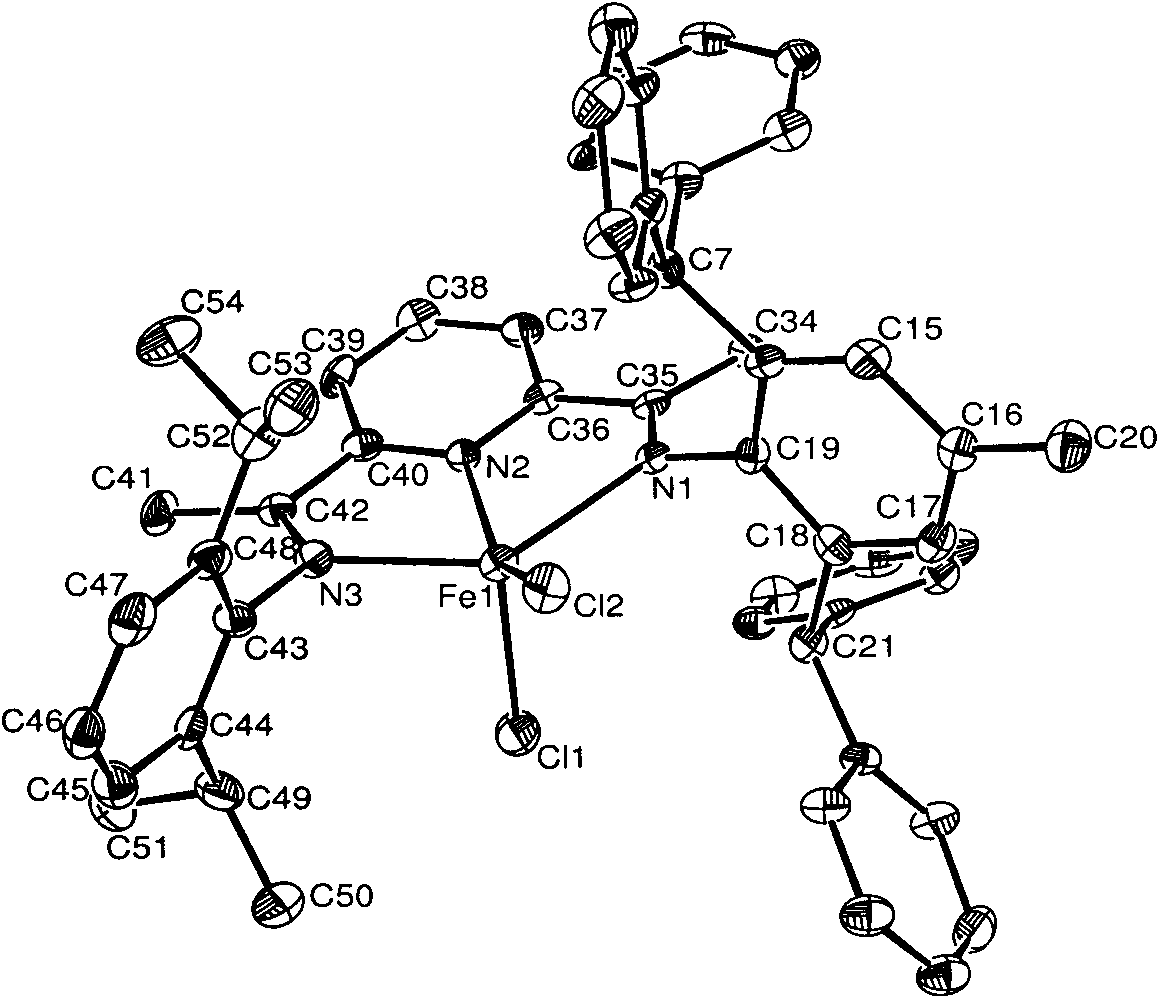
Asymmetric diimine pyridine iron or cobalt complex catalyst, and preparation method and application thereof
A technology of iron diimide pyridine and complexes, applied in the direction of iron organic compounds, cobalt organic compounds, etc., can solve problems such as limiting metal catalysts, and achieve the effects of high catalytic activity, high molecular weight, and strong tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, preparation complex Fe3 (in formula I, R1=i-Pr; R2=H; M=Fe)
[0058] 1. Preparation of 2-acetyl-6-(1-(2,6-benzhydryl-4-methylaniline) ethyl)pyridine: in 2,6-benzhydryl-4-methyl Toluene (60mL) solution of phenylaniline (3.48g, 8mmol) and 2,6-diacetylpyridine (1.30g, 4mmol) was added with catalyst equivalent (0.15g) of p-toluenesulfonic acid, and the reaction was refluxed for 6h. The solvent was removed, and the residue was subjected to alumina column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 20:1. Mixed solvent of ether and ethyl acetate, the fraction with Rf value of 1 / 2-1 / 3 (ie the second fraction) was collected, and the solvent was removed to obtain a yellow solid. Yield: 44%. Melting point: 134-136°C.
[0059] The structure confirmation data are as follows: FT-IR (KBr, cm -1 ): 3024, 2919, 2160, 2030, 1977, 1702, 1642 (C=N), 1493, 1466, 1365, 1235, 1118, 1076, 1031, 819, 765, 739, 698. 1 H NMR (400...
Embodiment 2
[0099] Embodiment 2, preparation complex Fe1 (in formula I, R1=Me; R2=H; M=Fe)
[0100] 1. The preparation of 2-acetyl-6-(1-(2,6-benzhydryl-4-methylaniline)ethyl)pyridine is the same as in Example 1.
[0101] 2. Preparation of ligand: 2-(1-(2,6-dimethylaniline) ethyl)-6-(1-(2,6-dibenzhydryl-4-methylaniline) ethyl ) pyridine (L1): 2-acetyl-6-(1-(2,6-dibenzhydryl-4-methylaniline) ethyl)pyridine (1.14g, 2.0mmol) and 2,6-bis A catalyst equivalent (300 mg) of p-toluenesulfonic acid was added to a solution of methylaniline (0.21 g, 2.0 mmol) in toluene (50 mL). Heated to reflux for 8h. The solvent toluene was removed, and the residue was subjected to alumina column chromatography with a solvent of petroleum ether and ethyl acetate at a volume ratio of 50:1. The elution fraction was detected by a thin-layer silica gel plate, and the developing solvent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 100:1, and the fraction with an Rf value of 2 / 3-1 / 3...
Embodiment 3
[0108] Embodiment 3, preparation complex Fe2 (in formula I, R1=Et; R2=H; M=Fe)
[0109] 1. The preparation of 2-acetyl-6-(1-(2,6-benzhydryl-4-methylaniline)ethyl)pyridine is as in Example 1.
[0110] 2. Preparation of ligand: 2-(1-(2,6-diethylaniline) ethyl)-6-(1-(2,6-dibenzhydryl-4-methylaniline) ethyl ) pyridine (L2): 2-acetyl-6-(1-(2,6-dibenzhydryl-4-methylaniline) ethyl)pyridine (1.14g, 2.0mmol) and 2,6-bis A catalytic equivalent of p-toluenesulfonic acid was added to a solution of ethylaniline (0.22, 2.0 mmol) in toluene (50 mL). Heated to reflux for 8h. The solvent toluene was removed, and the residue was subjected to alumina column chromatography with a solvent of petroleum ether and ethyl acetate at a volume ratio of 50:1. The elution fraction was detected by a thin-layer silica gel plate, and the developing solvent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 100:1, and the fraction with an Rf value of 2 / 3-1 / 3 (ie the third fracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com