Electrode for generation of hydrogen, and electrolysis method
A technology of hydrogen evolution electrode and electrode active material, which is applied in the field of salt electrolysis by ion exchange membrane method, which can solve the problems of reduction of electrode effective area, increase of electrolysis voltage, increase of liquid resistance, etc., and achieves suppression of cathode overvoltage rise and excellent resistance , The effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0040] (Example 1-1) (preparation of electrode 1)
[0041] A porous metal mesh (LW: 8.0, SW: 3.6, ST: 1.2 mm) made of nickel metal was used as the electrode substrate. The size of the expanded metal mesh is 20 mm x 20 mm x 1.2 mm thick. A nickel round rod with a diameter of 1.5 mm and a length of 80 mm was welded to the expanded metal plate as a power supply guide rod for energization.
[0042] Use No. 100 artificial corundum to perform sandblasting treatment on the surface of the substrate porous metal mesh with a pressure of 0.3 MPa. After the porous metal mesh was placed in acetone and degreased by ultrasonic cleaning, it was etched at 30° C. for 1 hour using a 10 wt % hydrochloric acid aqueous solution. The etched porous metal mesh was washed in running water for a day and night.
[0043] According to the platinum: cerium molar ratio of 25:75, chloroplatinic acid hexahydrate and cerium nitrate hexahydrate were dissolved and prepared into an aqueous solution containing 6...
Embodiment 1-2
[0044] (Example 1-2) (overvoltage measurement)
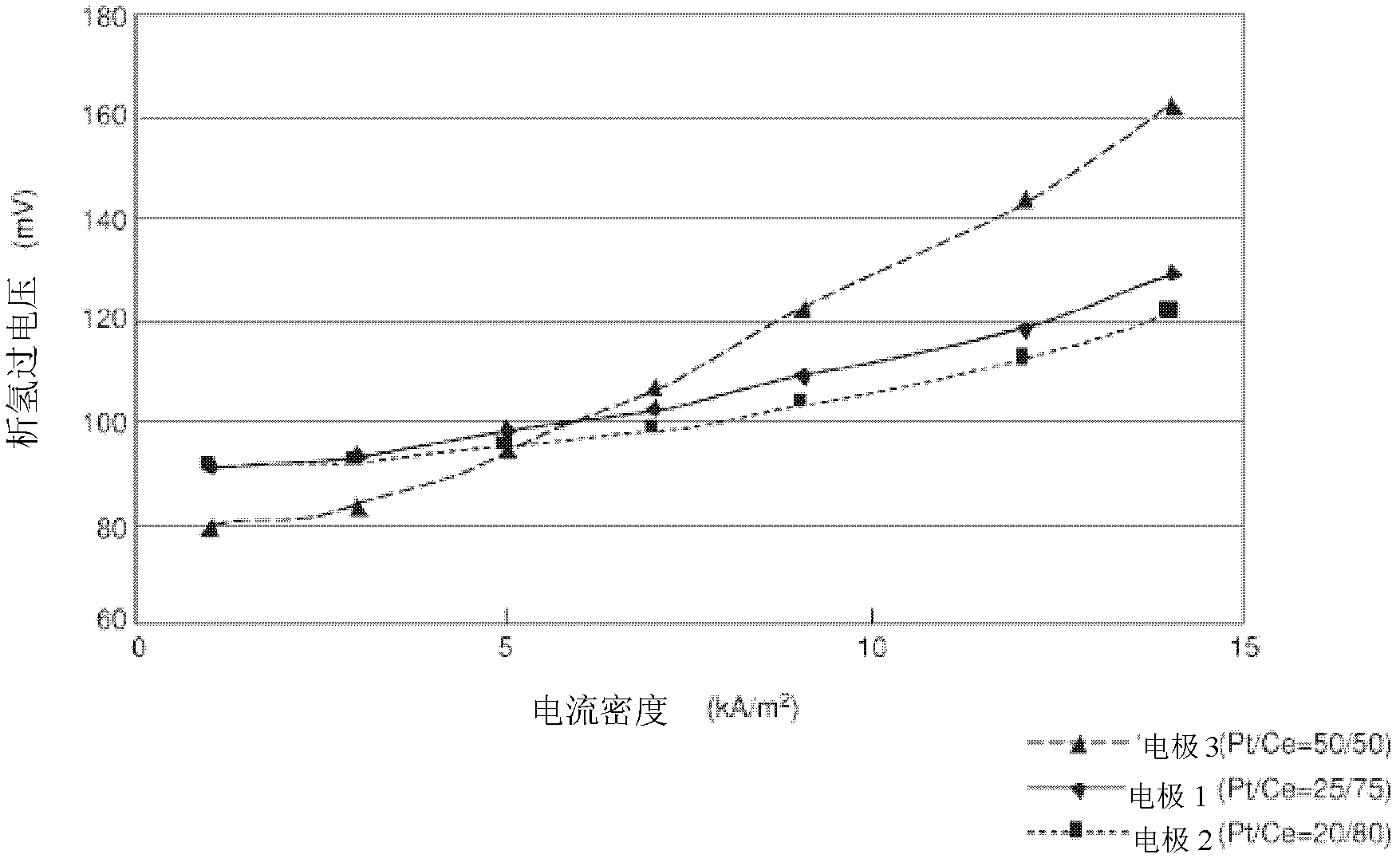
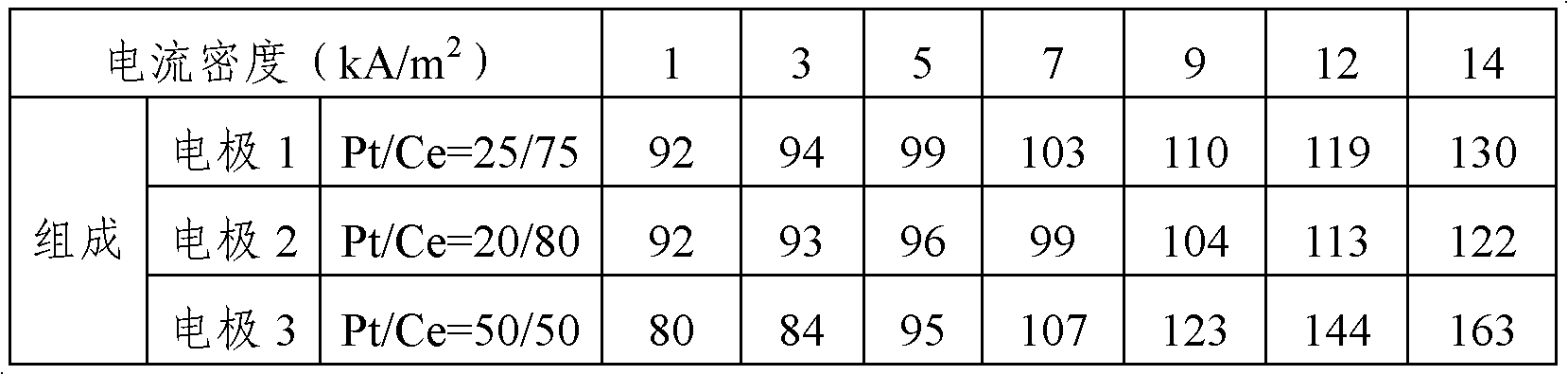
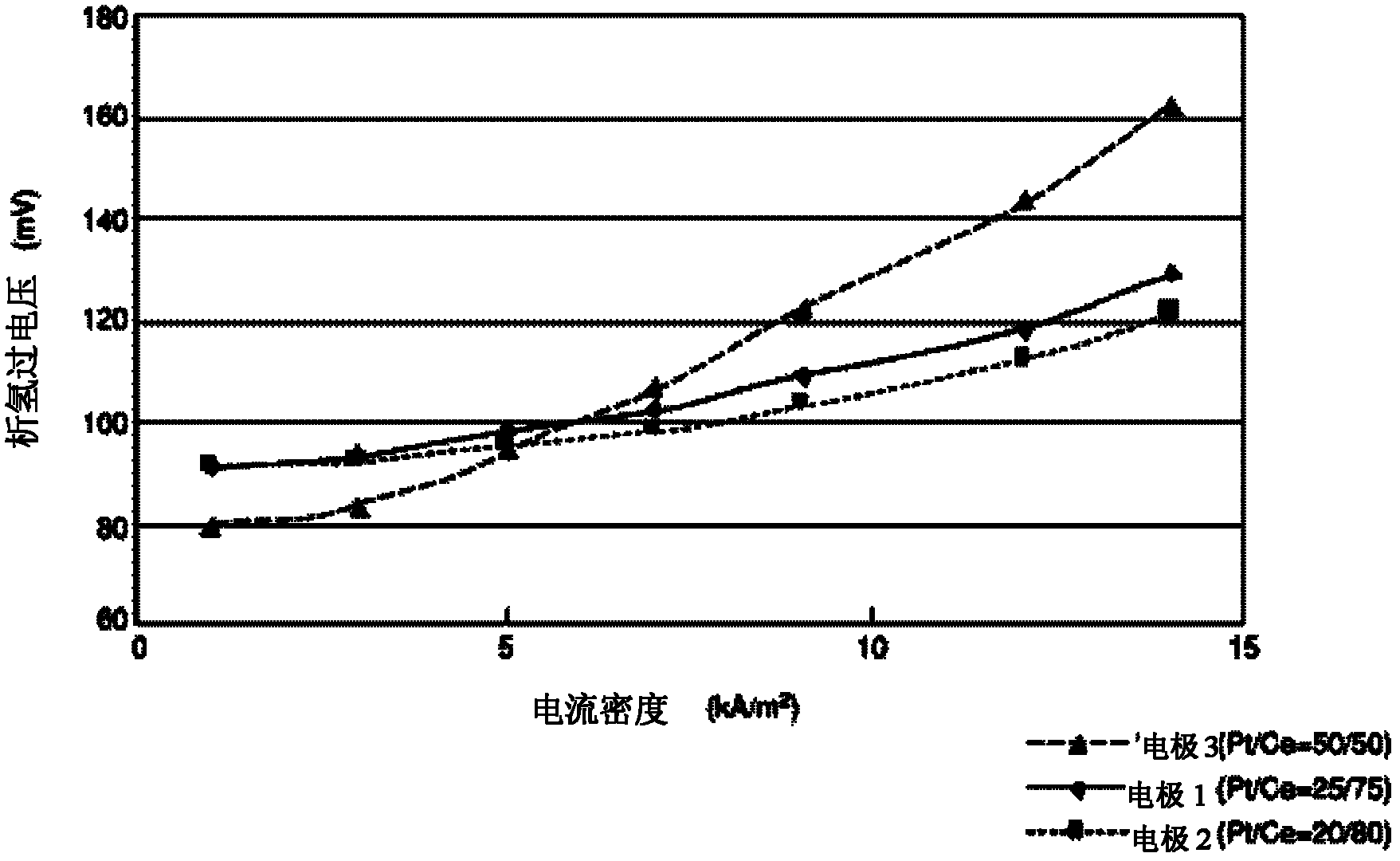
[0045] "Electrode 1" prepared in Example 1 only kept the surface and the back of the 20mm×20mm porous part, and the other part was sealed and used as the cathode. A 30mm×30mm×1mm thick nickel plate was used as the anode, 32wt% sodium hydroxide aqueous solution was used as the electrolyte, the distance between the cathode and the anode was 2cm, and the electrolysis experiment was carried out at 80°C. A partially exposed platinum wire wrapped in a tube made of PFA resin (tetrafluoroethylene-perfluoroalkyl vinyl ether copolymer) was wound around the cathode as a reference electrode. Be careful that the exposed part of the platinum wire does not come into contact with the cathode. The hydrogen evolution overvoltage of the cathode is measured by the constant current method, and the error caused by the liquid resistance due to the voltage drop when the current is cut off is corrected by the current gradient method.
[0046] The rela...
Embodiment 2
[0047] (Example 2) (preparation of electrode 2 and measurement overvoltage)
[0048] As the electrode base, the same nickel metal expanded metal plate and power supply guide rod as in Example 1 were used, and the same surface treatment as in Example 1 was performed.
[0049] According to the platinum: cerium molar ratio of 20:80, dinitrosodiammine platinum and cerium nitrate hexahydrate were dissolved and formulated into an aqueous solution containing 6 wt% nitric acid, which was used as a coating solution for electrode active materials. The amount of platinum metal in the coating solution was 7.5 g / L. The prepared coating solution was coated on both sides of the porous metal mesh, dried at 100° C. for 10 minutes, and then calcined in an electric furnace at 470° C. for 20 minutes. The coating operation (coating, drying, heating) of this electrode active material was repeated 10 times, and "electrode 2" was produced. With the method identical with embodiment 1-2, measure the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com