Compound artemisinin piperaquine pellet and preparation method thereof
A technology of compound artemisinin and piperaquine pellets, which is applied in the field of medicinal chemistry, can solve the problems of poor molding and roundness of antimalarial pellets, difficulties in the production of antimalarial drugs for children, and unstable dissolution time, etc. Solubilization, dissolution rate is not affected, good molding and roundness effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention also provides a preparation method of compound artemisinin-like piperaquine pellets, comprising:
[0035] Step 1. Weigh 20-100 parts by weight of artemisinin compounds, 100-500 parts by weight of piperaquine phosphate, 50-300 parts by weight of lactose, 50-300 parts by weight of microcrystalline cellulose, 10-100 parts by weight of cross-linked polymer Vinylpyrrolidone, 0-50 parts by weight of sodium carboxymethyl starch and 1-20 parts by weight of copovidone are mixed;
[0036] Step 2, dissolving 0.05-5 parts by weight of a solubilizer in water as a wetting agent, and preparing pellets by extrusion spheronization or centrifugal pelletization.
[0037] The preparation method of another kind of compound artemisinin-like piperaquine pellets provided by the present invention comprises:
[0038] Step 1. Weigh 30-60 parts by weight of artemisinin compounds, 250-450 parts by weight of piperaquine phosphate, 100-250 parts by weight of lactose, 80-260 par...
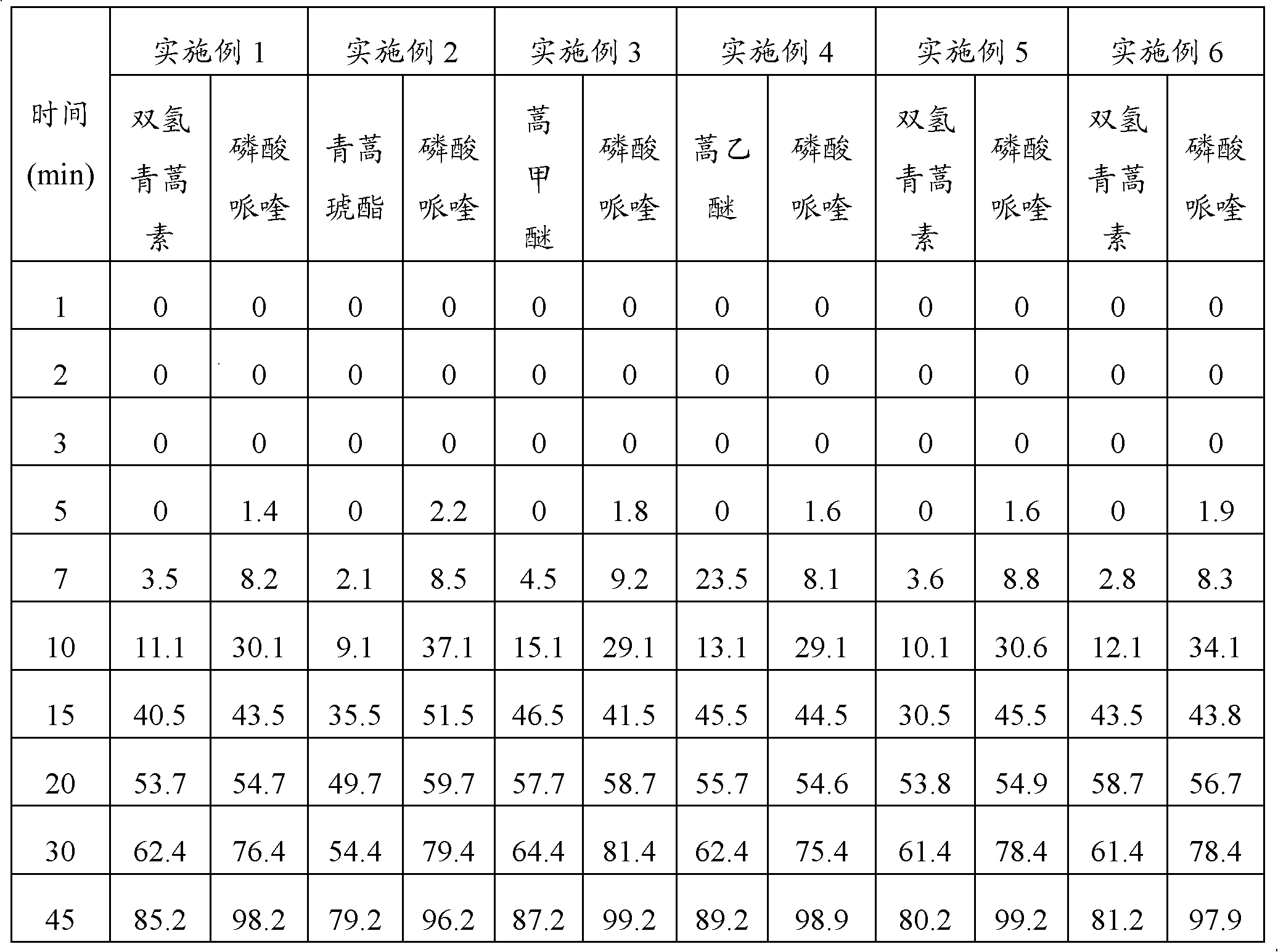
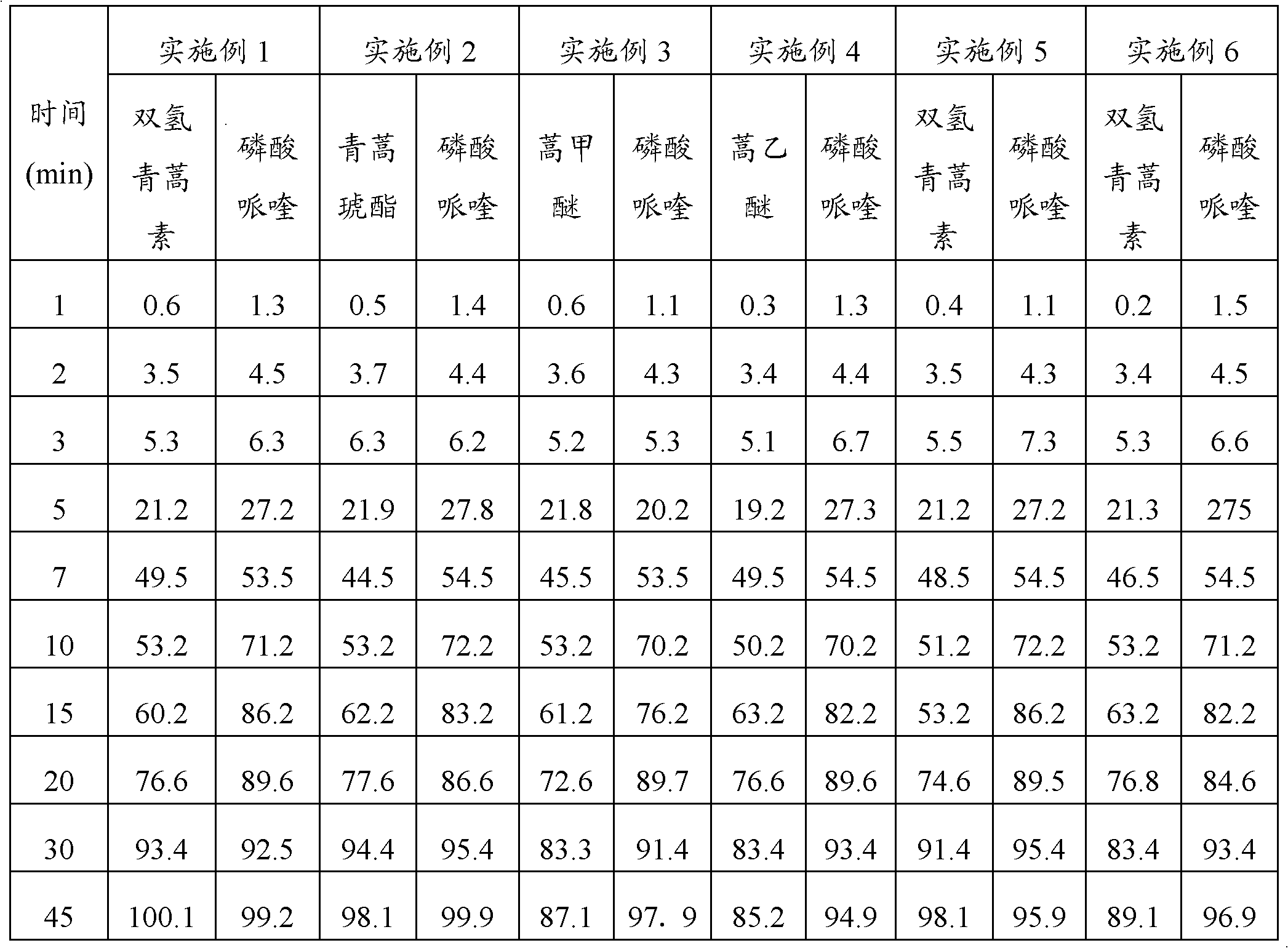
Embodiment 1
[0047] Embodiment 1: Preparation method of the present invention prepares compound artemisinin-like piperaquine pellets
[0048] 1. Prescription
[0049] Dihydroartemisinin 20g
[0050] Piperaquine Phosphate 480g
[0051] Lactose 164g
[0052] Microcrystalline Cellulose 140g
[0053] Cross-linked polyvinylpyrrolidone 10g
[0054] Copovidone 8g
[0055] Sodium Lauryl Sulfate 1g
[0056] Pure water 200g
[0057] Gastric-soluble coating powder 170g
[0058] Aspartame 2% (total weight of coating material)
[0059] 2. Preparation method
[0060] Pass the original and auxiliary materials through a 100-mesh sieve, weigh dihydroartemisinin, piperaquine phosphate, lactose, microcrystalline cellulose, cross-linked polyvinylpyrrolidone and copovidone according to the prescription amount, and then mix them evenly to obtain a mixed powder; Dialkyl sodium sulfate is dissolved in water as a wetting agent; take 15% of the mixed powder, put it in a centrifugal pelletizer, and spray i...
Embodiment 2
[0062] Embodiment 2: Preparation method of the present invention prepares compound artemisinin-like piperaquine pellets
[0063] 1. Prescription
[0064] Artesunate 40g
[0065] Piperaquine Phosphate 400g
[0066] Lactose 300g
[0067] Microcrystalline Cellulose 300g
[0068] Cross-linked polyvinylpyrrolidone 100g
[0069] Sodium carboxymethyl starch 50g
[0070] Copovidone 20g
[0071] Span-40 2g
[0072] Pure water 300g
[0073] Gastric-soluble coating powder 320g
[0074] Aspartame 1% (accounting for the total weight of coating materials)
[0075] 2. Preparation method
[0076] Pass the raw materials and auxiliary materials through a 100-mesh sieve, weigh artesunate, piperaquine phosphate, lactose, microcrystalline cellulose, cross-linked polyvinylpyrrolidone, sodium carboxymethyl starch and copovidone according to the prescription amount and mix them evenly to obtain Mixed powder; use Span-40 dissolved in water as a wetting agent; put the mixed powder in a wet m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com