Method for synthesizing florfenicol midbody RT0131
A technology of RT0131 and florfenicol, which is applied in the field of synthesizing florfenicol intermediates, can solve the problems of high requirements on container and reaction conditions, low yield of finished products, inconvenient operation, etc., and achieve reduced production costs, high conversion efficiency, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
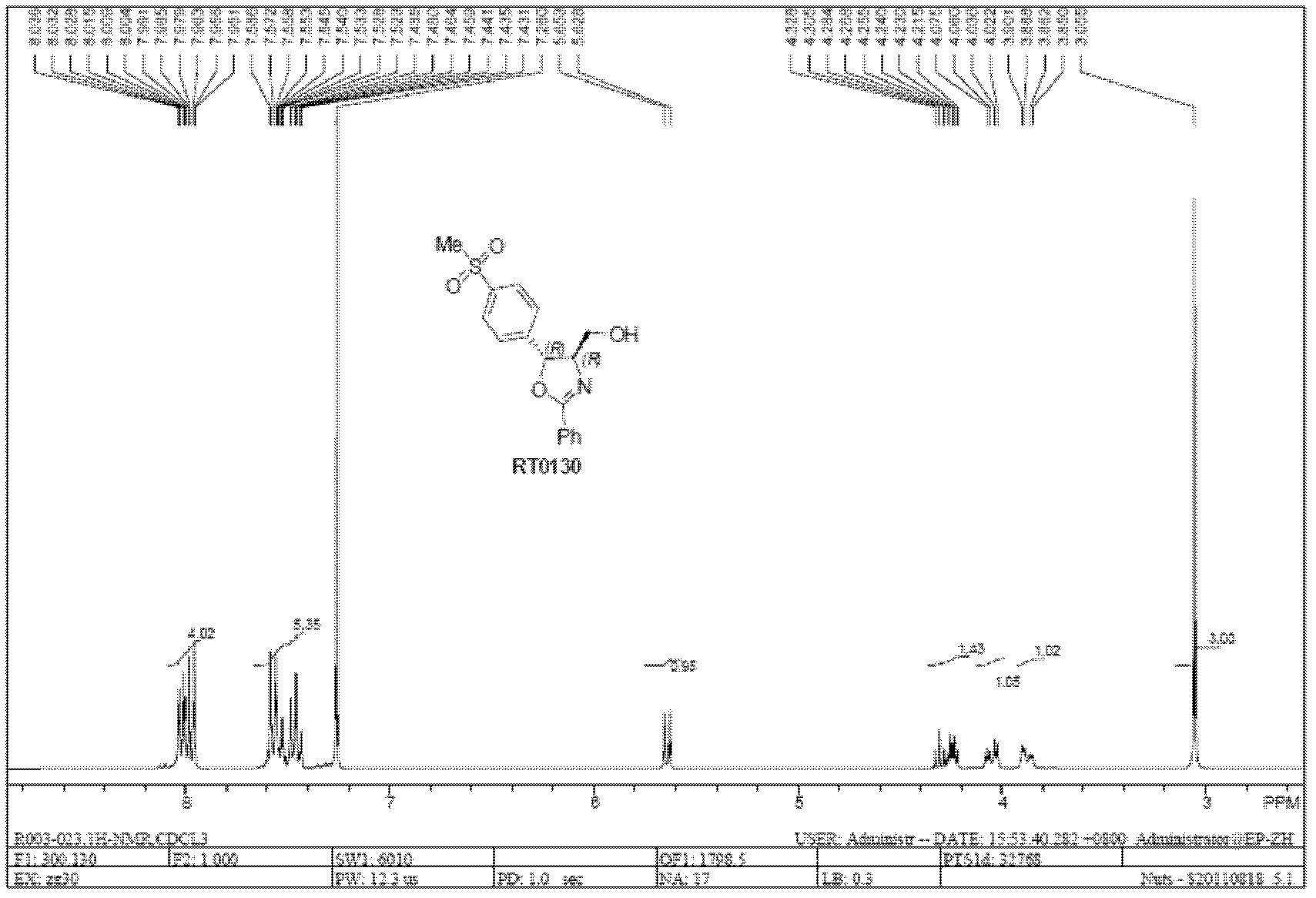
[0087] Example 1: RT0130-A generates RT0130-SM through alkaline hydrolysis deamidation
[0088] Add 50.0 g (0.14 mol) of solid RT0130-A into 373 ml of sodium hydroxide solution (1.5 mol / L, 0.56 mol), stir rapidly at 60°C for 2.5 hours, cool to room temperature, add 500 ml of methyl tert-butyl ether for extraction , the organic phase was separated, concentrated, and dried in vacuo to obtain RT0130-SM as a white solid.
[0089] Feeding ratio (molar ratio):
[0090] RT0130-A:NaOH=1:4
[0092] The chemical reaction formula is as follows:
[0093]
Embodiment 2
[0094] Example 2: RT0130-SM reacts and condenses with phenylacetonitrile to generate RT0130
[0095]Dissolve 24.5 grams (0.10 moles) of RT0130-SM and 20.7 grams (0.15 moles) of anhydrous potassium carbonate in 46 milliliters of glycerol, stir and heat to 115°C, and dissolve 19.0 grams of benzonitrile (PhCN) within half an hour Add the above white suspension solution dropwise, keep warm and stir rapidly for 18 hours, TCL detects that the reaction is complete, cool to room temperature, add 400 ml of ice water to dilute and stir evenly, keep warm at 4-10°C for 1 hour, filter, wash with ice water, Drying in vacuo yielded 31.5 g (0.095 mol) of RT0130 as a white solid.
[0096] Feeding ratio (molar ratio)
[0097] RT0130-SM: benzonitrile: potassium carbonate = 1.00: 1.85: 1.50
[0098] Protic Solvent: Glycerol
[0099] The chemical reaction formula is as follows:
[0100]
Embodiment 3
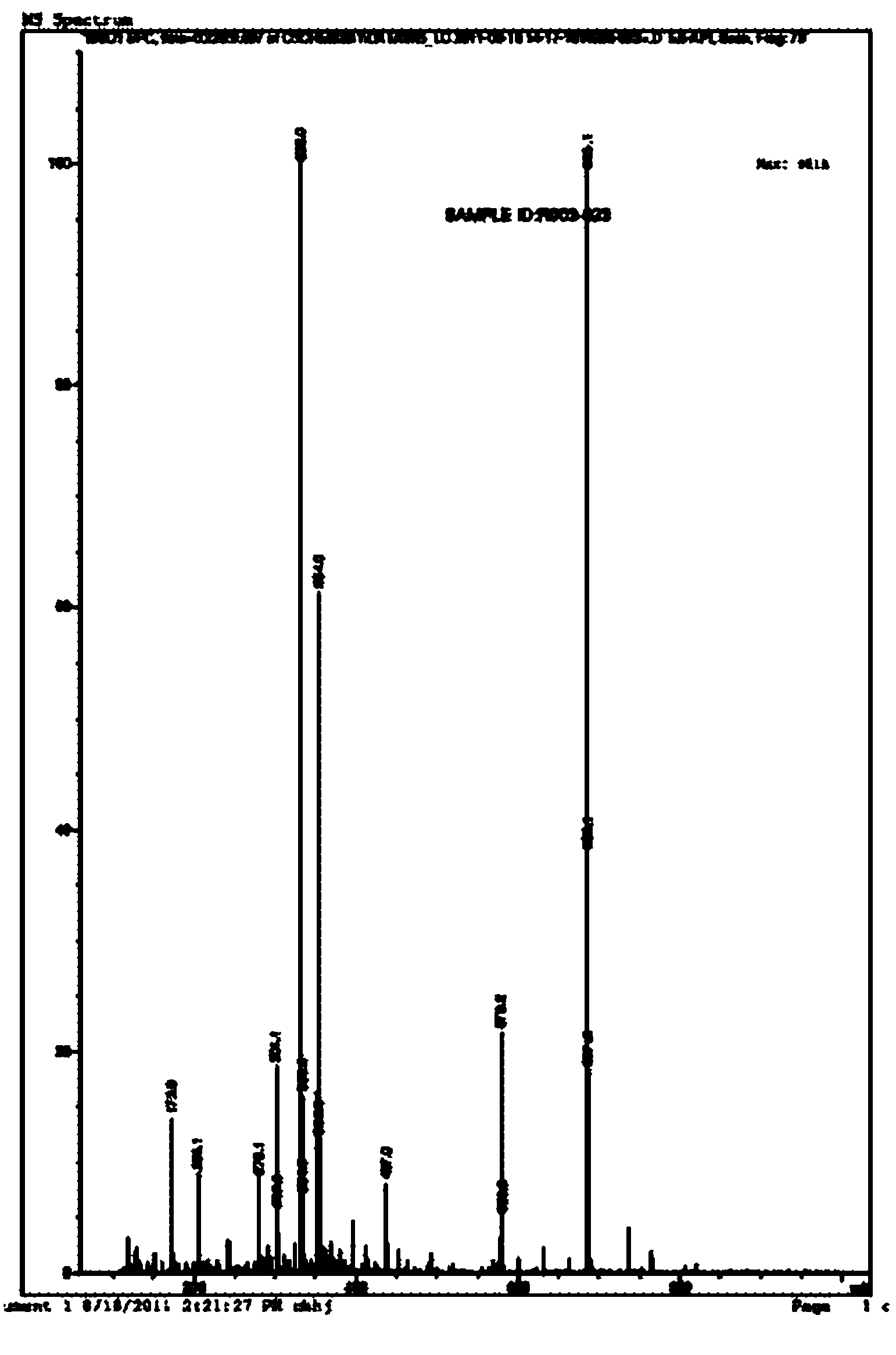
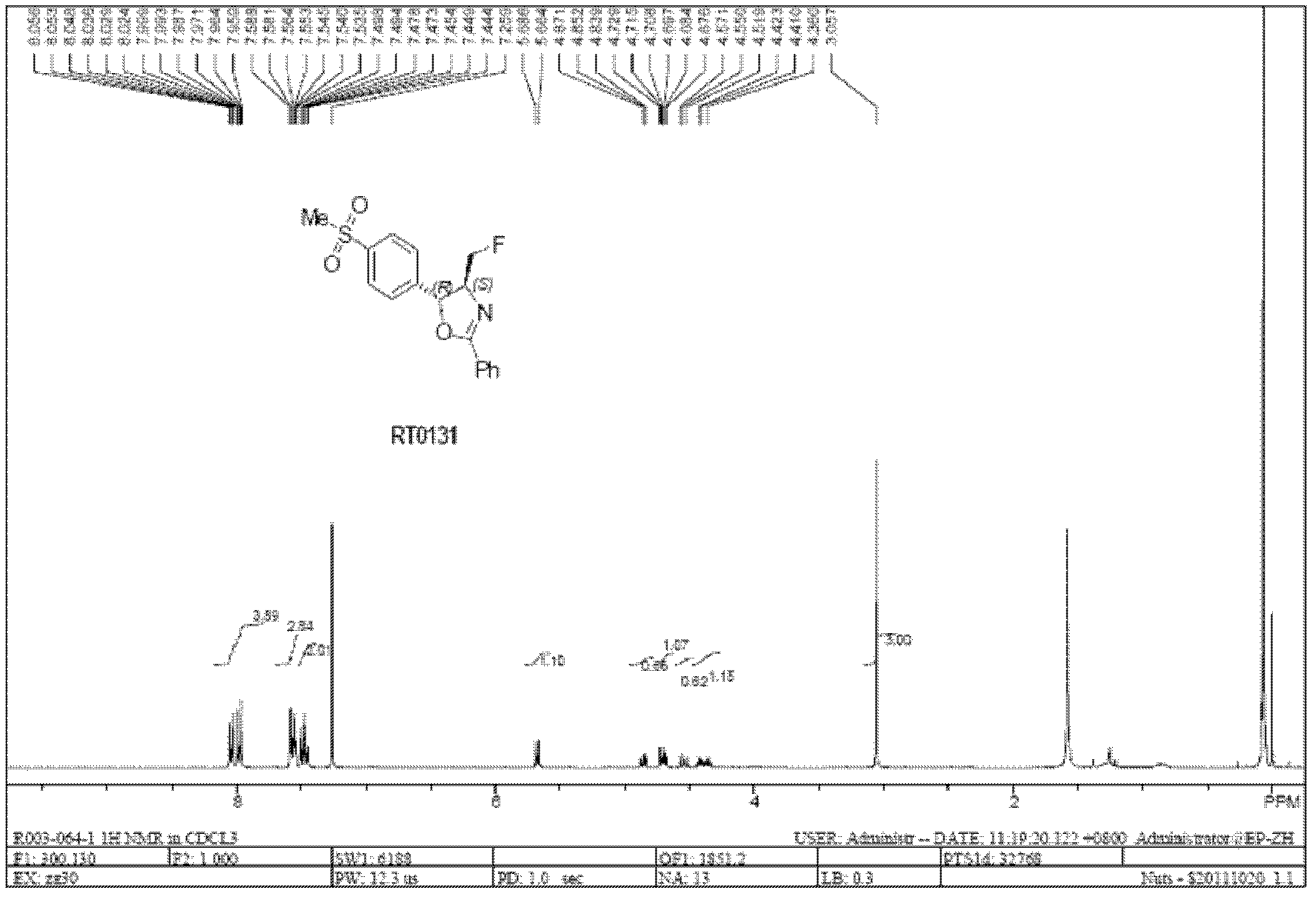
[0101] Example 3: RT0130 was fluorinated by XtalFluor-E to generate RT0131
[0102] 31.5 g (0.095 mol) of solid RT0130 were dissolved in 315 mL of dry dioxane. Slowly add potassium carbonate (0.28 mole) and solid XtalFluor-E (0.28 mole) reagent under nitrogen protection, after stirring well, slowly rise to room temperature and stir reaction for 24 hours, TLC (Thin Layer Chromatography, thin plate chromatography) detects reaction Finish. The reaction liquid was slowly poured into ice-cold potassium bicarbonate aqueous solution (0.1 mol / L), the organic phase was separated, decolorized with 5% activated carbon, dried, and concentrated to obtain 28.5 g (0.090 mol) of RT0131 as a light yellow-brown solid.
[0103] Feeding ratio (molar ratio)
[0104] RT0130:K 2 CO 3 : XtalFluor-E reagent = 1.00: 2.95: 2.95
[0105] Solvent: Dioxane
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com