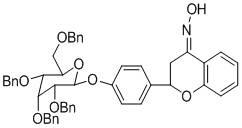
Preparation method and application of 2-(4-beta-D-allose pyranoside-phenyl)-2,3-dihydroquinoline-4(1H) and 2-(4-(2,3,4,6-tetrabenzyl)-beta-D-allose pyranoside-phenyl)-2,3-benzodihydropyran
A technology of allopyranoside and dihydrobenzopyran, which is applied in the field of tofu glucoside derivatives, and can solve the problems of less side reactions, affecting the popularization and application of drugs, and good curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
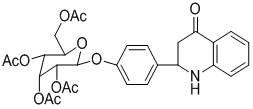
[0017] Example 1: 2-(4- β-D -Allopyranoside-phenyl)-2,3-dihydroquinoline-4(1 H )-Synthesis of ketone (3a)
[0018]
[0019] In a 50mL round bottom flask, 0.6mmol L-proline was dissolved in 20mL absolute anhydrous methanol, and then 2mmol of tofu glucoside and 2mmol of o-aminoacetophenone were added successively, and the reaction was stirred at room temperature for 5 days. After the reaction, the crude product was separated and purified by column chromatography (dichloromethane:methanol=8:1) to obtain yellow-green solid 3a. Yield: 60%, m.p. 115-117℃; 1 H NMR (400MHz, DMSO-d 6 ) δ : 6.61~7.59(m, 8H), 5.15(d, J =8.0 Hz, 1H), 5.07(d, J =6.4 Hz, 1H), 4.95(d, J =3.6 Hz, 1H), 4.65~4.71(m, 2H), 4.49(t, J =4.0 Hz, 1H), 3.66~3.92(m, 3H), 3.36~3.47(m, 4H), 2.59~2.85(m, 2H); IR (KBr) ν : 3382, 2920, 1657, 1609, 1507, 1479, 1329, 1082,1037, 912, 836, 763; HRMS (ESI) calcd for C 21 h 23 NO 7 [M+H] + : 402.1547, found 402.1562; [M+Na] + : 424.1367, found 424.1368.
example 2
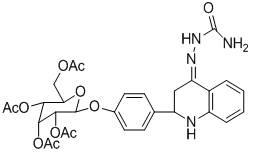
[0020] Example 2: 2-(4- β - D -Allopyranoside-phenyl)-2,3-dihydroquinoline-4(1 H )-Synthesis of methoxylamine (3b)
[0021]
[0022] Add 2mmol of 3a dissolved in 10mL of ethanol to a 25mL round bottom flask, then add 2mmol of methoxylamine hydrochloride (neutralized with dilute NaOH solution before addition) 2mmol under stirring, and reflux for 2h after the addition. After the reaction stopped, the solvent was spinned off, dissolved in ethyl acetate, washed with water, and the extract was washed with anhydrous Na 2 SO 4 Drying, rotary evaporation to remove the solvent, the crude product was separated and purified by column chromatography (dichloromethane: methanol = 8:1) to obtain yellow solid 3b, yield: 90%, m.p. 114-116°C; 1 H NMR (400MHz, DMSO-d 6 ) δ: 7.64(d, J =8.0 Hz, 1H), 7.35(d, J =8.4 Hz, 2H), 7.10(t, J =8.0 Hz, 1H), 7.00(d, J =8.0 Hz, 2H), 6.77(d, J =8.0 Hz, 1H), 6.59(t, J =8.0 Hz, 1H), 6.40(s, 1H), 5.07~5.12(m, 2H), 4.95(d, J =2.0 Hz, 1H), 4.66(d, ...
example 3
[0023] Example 3: 2-(4- β - D -Allopyranoside-phenyl)-2,3-dihydroquinoline-4(1 H )-Synthesis of Hydroxylamine (3c)
[0024] Add 2mmol of 3a dissolved in 10mL of ethanol to a 25mL round bottom flask, then add 2mmol of hydroxylamine hydrochloride (neutralized with dilute NaOH solution before addition) 2mmol under stirring, and reflux for 2h after the addition is complete. After the reaction stopped, the solvent was spinned off, dissolved in ethyl acetate, washed with water, and the extract was washed with anhydrous Na 2 SO 4 Drying, rotary evaporation to remove the solvent, the crude product was separated and purified by column chromatography (dichloromethane:methanol=8:1) to obtain a yellow solid 3c, yield: 80%, m.p. 118~120°C; 1 H NMR (400MHz, DMSO-d 6 ) δ : 10.86(s, 1H), 7.64(d, J =8.0 Hz, 1H), 7.35(d, J =8.4 Hz, 2H), 7.06(t, J =7.6 Hz, 1H), 7.00(d, J=8.2 Hz, 2H), 6.76(d, J =8.2 Hz, 1H), 6.59(t, J =7.6 Hz, 1H), 6.31(s, 1H), 5.07~5.12(m, 2H), 4.97(s, 1H), 4.68(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com