Macromolecule-cis-platinum compound, preparation method and application thereof
A complex and macromolecular technology, which is applied in the field of macromolecular-cisplatin complexes, can solve the problems of low effective binding rate of cisplatin in the complex drug load, large amount of carrier, and low release rate, etc., to achieve increased effective binding rate, improved release behavior, and reduced drug dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation and structure characterization of γ-polyglutamic acid
[0048] (1) Degradation conditions
[0049] Weigh an appropriate amount of γ-polyglutamic acid, add distilled water to dissolve to obtain a 2% solution, directly adjust the pH to 2-3 with concentrated hydrochloric acid, react at 121°C and 0.1MPa for 20 minutes, cool in an ice bath, and use 1mol / L Adjust the pH to 7-8 with NaOH, terminate the reaction, purify by dialysis, and freeze-dry to obtain small molecule γ-polyglutamic acid.
[0050] (2) Structural characterization
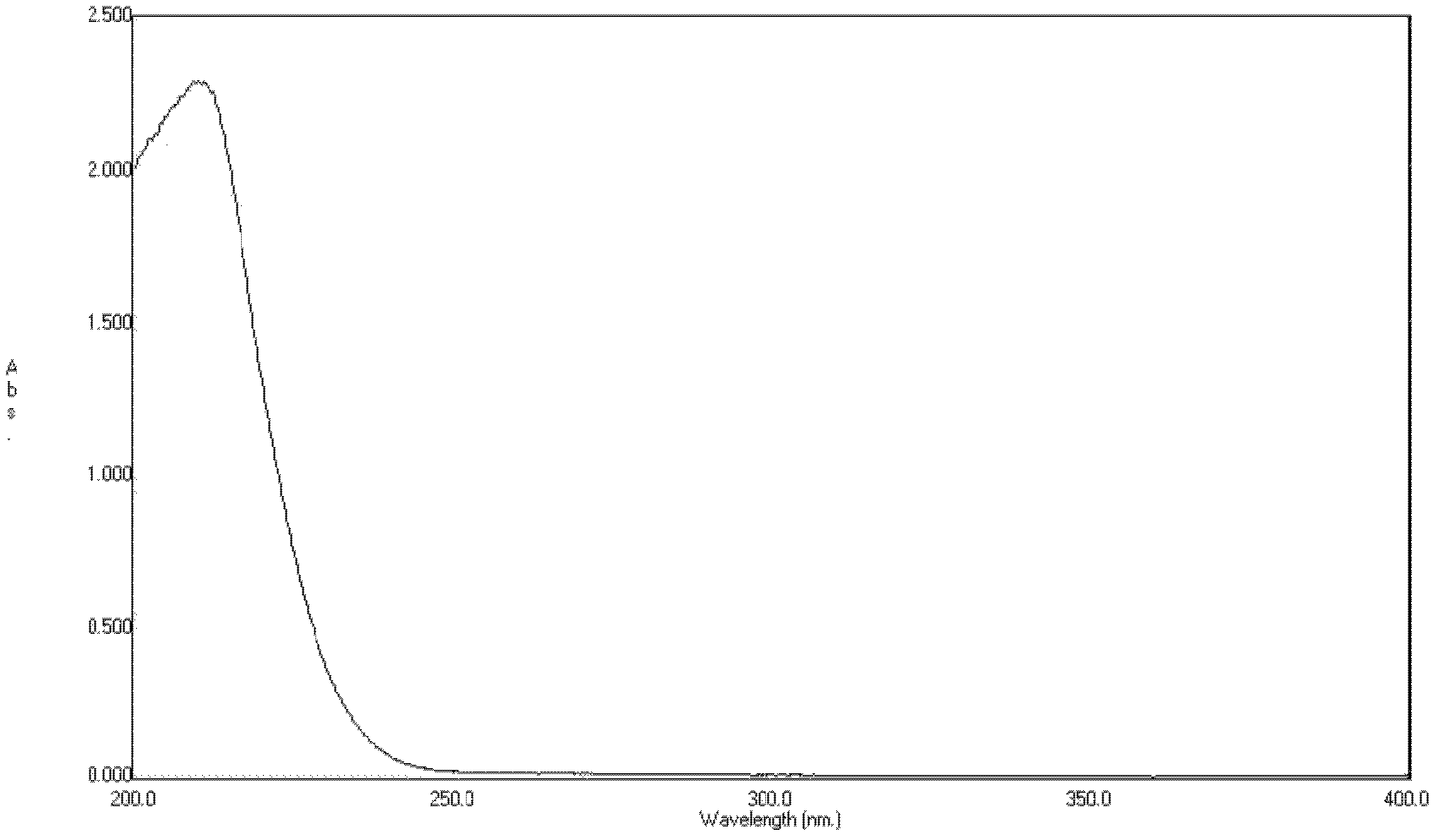
[0051] Ultraviolet scanning spectrum analysis Take an appropriate amount of dry pure γ-polyglutamic acid, add distilled water to dissolve, and scan in the range of 200-400nm, the results are shown in figure 1 .
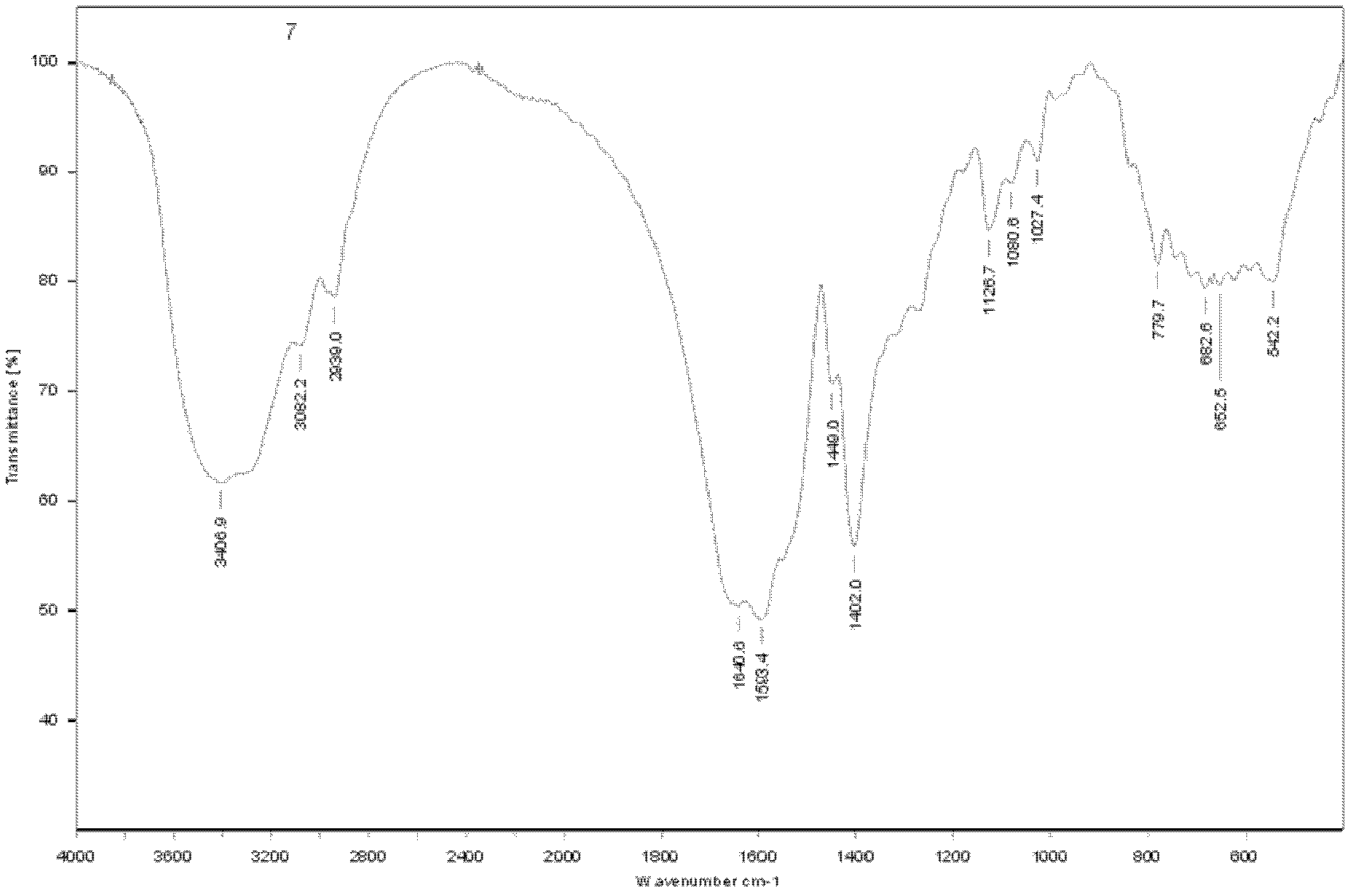
[0052] Infrared spectrum analysis Take an appropriate amount of dry pure γ-polyglutamic acid, grind it with a small amount of KBr, and press it into a tablet to prepare a sample. At room temperature, scan in the range of 40...
Embodiment 2
[0055] Synthesis and Structure Characterization of γ-Polyglutamic Acid-Citrate Polymer
[0056] Precisely weigh 129mg of small molecule γ-polyglutamic acid, add 10ml of water to dissolve, stir in ice bath for a while, add EDCI 384mg, NHSS 60mg, continue stirring in ice bath for a while, take 210mg of citric acid and dissolve in appropriate amount of distilled water to adjust pH To neutrality, slowly add it dropwise to the above reaction solution, stir evenly, adjust the pH to 7.5-8.0, stir overnight at room temperature, dialyze against deionized water for 24 hours, and then freeze-dry to obtain the polymer.
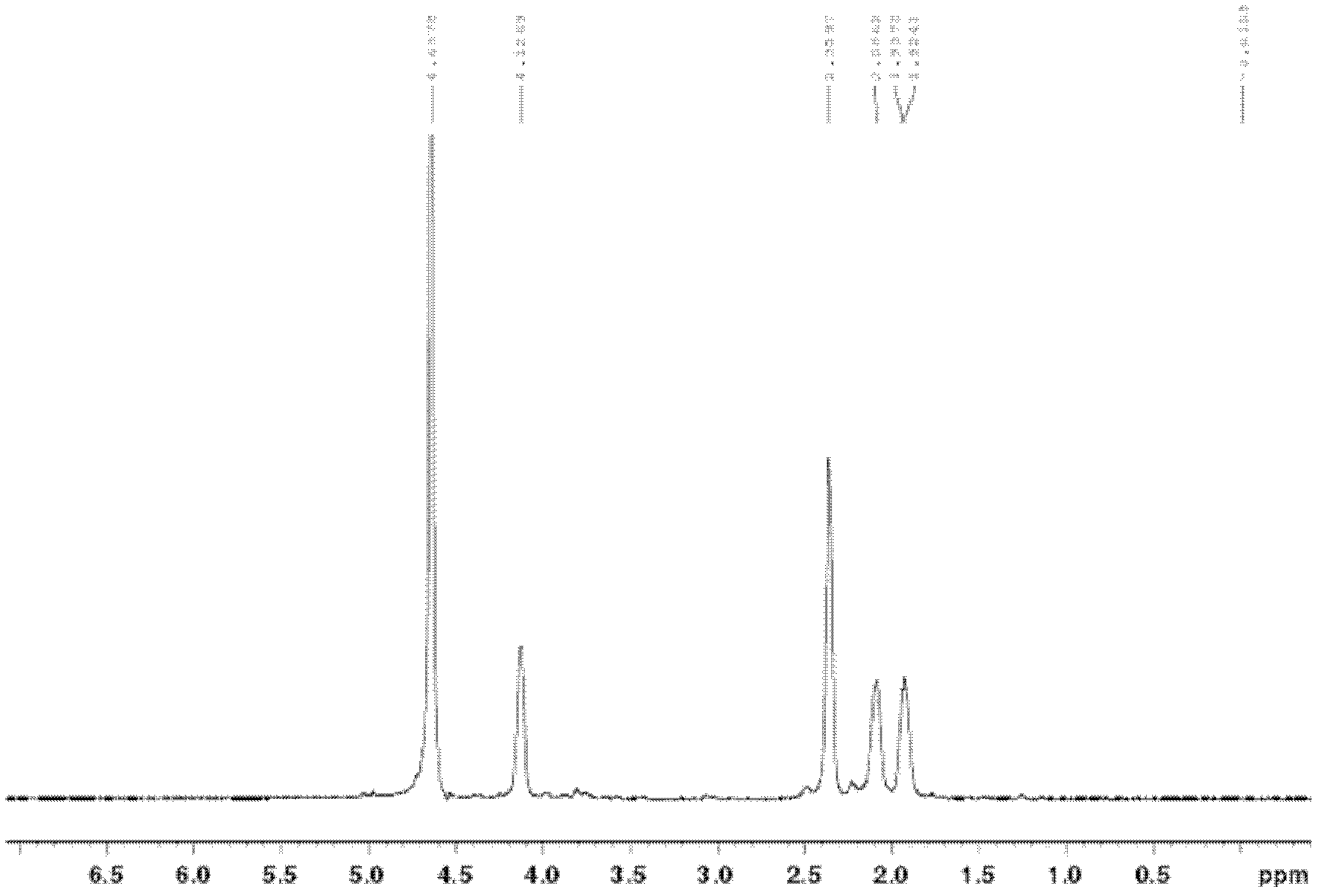
[0057] Structural characterization Take an appropriate amount of dry pure polymer of γ-polyglutamic acid-citric acid, dissolve in D 2 In O, H NMR spectroscopy ( 1 HNMR) detection, see the results Figure 4 .
Embodiment 3
[0059] Preparation of γ-polyglutamic acid citrate-cisplatin complex
[0060] Preparation of γ-polyglutamic acid citrate-cisplatin complex Precisely weigh 60 mg of cisplatin, 68 mg of silver nitrate and 100 ml of water to dissolve, react at room temperature in the dark for 24 hours, centrifuge, take the supernatant and filter it through a 0.45 μm filter membrane, collect The filtrate was placed in a 250ml three-necked bottle, and an equivalent amount of the above polymer was added, stirred to dissolve, adjusted to pH 7.5-8.0, reacted at room temperature for 48 hours, dialyzed against deionized water for 24 hours, and then freeze-dried to obtain the drug-loaded complex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com