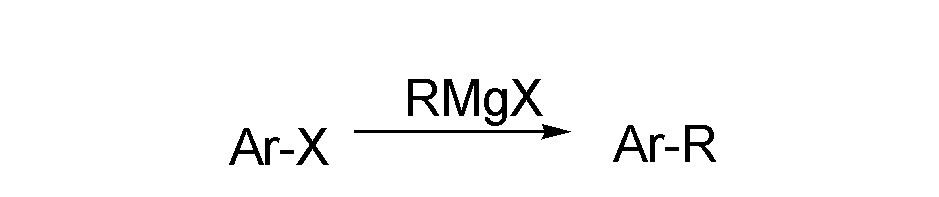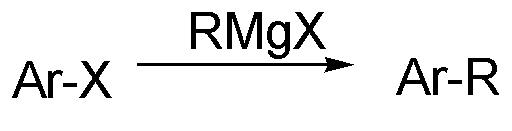Grignard reagent and aryl halide coupled alkyl introduction method by metal catalysis
A metal catalyzed Grignard and metal catalyst technology, applied in the field of aromatic hydrocarbon alkylation, can solve the problems of high alkylation reaction temperature, containing many by-products, complicated catalyst preparation, etc., and achieves high conversion rate, stable yield, The effect of suppressing isomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing 2-cyclopropylpyridine, characterized in that the specific preparation steps are as follows:
[0027] (1) Add cuprous oxide 1.9g (0.03eq), 2-chloropyridine 50g (0.44mol, 1eq), 1,4-dioxane 50mL (1mL / g) successively into a 2L reaction flask, and cool down to - 10±2°C.
[0028] (2) Add 462 g (1.0 eq, 10%) of cyclopropylmagnesium chloride Grignard reagent dropwise into the reaction flask, after the drop is complete, keep warm at -10±2° C. until the HPLC detection reaction ends.
[0029] (3) Add 100 mL (2 mL / g) of saturated ammonium chloride solution to the reaction system to terminate the reaction.
[0030] (4) Extract the system obtained in step (3) once with 100 mL (2 mL / g) of 1,4-dioxane, separate the liquids to obtain an organic phase, concentrate the organic phase until there is no fraction, and obtain 35.68 g of the product with a yield of 68 %, HPLC purity 99.0%.
[0031] The NMR data of the resulting product is as follows: H-NMR: (300MHZ, CD...
Embodiment 2
[0033] A method for preparing 3-methylindole, characterized in that the specific preparation steps are as follows:
[0034] (1) Add 108g (0.1eq) of lithium chloride, 5kg (25.5mol, 1eq) of 3-bromoindole, and 50kg (10mL / g) of anisole to a 200L reactor in sequence, and cool down to 10±2°C.
[0035] (2) Add 56 kg (2.0 eq, 15%) of methylmagnesium bromide Grignard reagent dropwise into the reaction kettle, after dropping, keep warm at 10±2° C. until the HPLC detection reaction ends.
[0036] (3) Add 32 kg (6 mL / g) of saturated ammonium chloride solution to the reaction system to terminate the reaction.
[0037] (4) Extract the system obtained in step (3) once with 30 kg (6 mL / g) of anisole, separate the liquids to obtain an organic phase, and concentrate the organic phase until there is no fraction to obtain 2.2 kg of the product, with a purity of 98.9% and a yield of 66%. .
[0038] The NMR data of the resulting product is as follows: H-NMR: (300MHZ, CDCl3), δ0.57&0.32 (H on cycl...
Embodiment 3
[0040] A method for preparing 2-isopropylpyridine, characterized in that the specific preparation steps are as follows:
[0041] (1) Add 1.9kg (0.06eq) of anhydrous manganese chloride, 40kg (253mol, 1.0eq) of 2-bromopyridine, and 178kg (5mL / g) of tetrahydrofuran to a 1000L reactor, and cool down to 0±2°C.
[0042] (2) Add 176 kg (1.3 eq, 12%) of isopropylmagnesium chloride Grignard reagent dropwise to the reaction kettle, after dropping, keep warm at 0±2° C. until the HPLC detection reaction ends.
[0043] (3) Add 170 kg (4 mL / g) of saturated ammonium chloride solution to the reaction system to terminate the reaction.
[0044] (4) The system obtained in step (3) was extracted once with 143kg (4mL / g) tetrahydrofuran, separated to obtain an organic phase, and the organic phase was concentrated to no fraction to obtain 23.5kg of product with a purity of 98.9% and a yield of 77%.
[0045] The NMR data of the resulting product is as follows: H-NMR: (300MHZ, DMSO), δ1.29 (H of two ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com