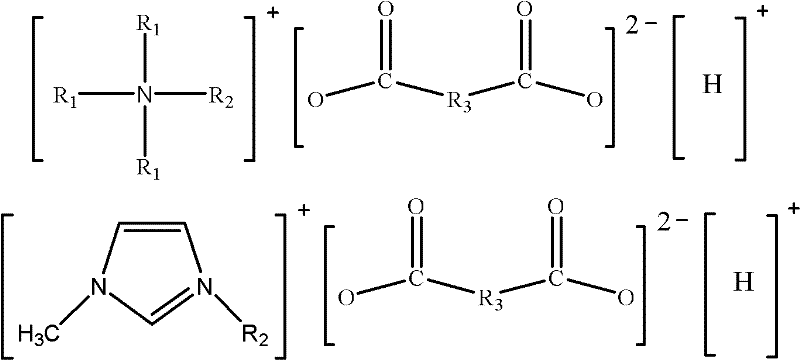
Dicarboxylic hydrogen salt ionic liquid with asymmetric chemical structure and weak acidity, and preparation method thereof
A dicarboxylic acid hydrogen salt, ionic liquid technology, applied in the preparation of carboxylate, the preparation of organic compounds, organic chemistry and other directions, can solve the problems of many reaction steps, complex synthesis process, high melting point, and achieve product yield and High purity, cheap and easy-to-obtain raw materials, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
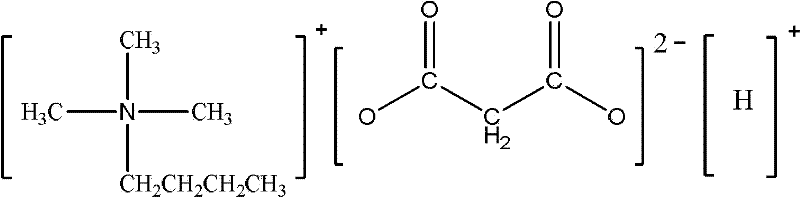
[0017] Embodiment 1: trimethylbutyl quaternary ammonium hydrogen malonate ionic liquid
[0018] Add 15.12g (0.1mol) of trimethylbutylammonium chloride, 14.22g (0.1mol) of potassium hydrogen malonate and 15g of solvent butanol into a 250mL reaction flask respectively, react for 30 seconds under 240W microwave, take out and stir Mix well, then put it into the microwave to react for 15 seconds, take it out and stir well, repeat the last step 3 times. After the reaction was completed, filter while it was hot, and the filtrate was rotary evaporated to remove and recover most of the solvent butanol, and vacuum-dried at 70°C for 48 hours to obtain the product trimethylbutyl quaternary ammonium hydrogen malonate ionic liquid, and the product yield was 95% %, chloride ion concentration 620ppm. Its chemical structural formula is:
[0019]
[0020] Characterization results: elemental analysis theoretical value C: 54.77%, H: 9.65%, N: 6.38%, measured value C: 54.75%, H: 9.61%, N: 6.4...
Embodiment 2
[0021] Embodiment 2: trimethylbutyl quaternary ammonium hydrogen succinate ionic liquid
[0022] Add 15.12g (0.1mol) of trimethylbutylammonium chloride, 15.62g (0.1mol) of potassium hydrogen succinate, and 30g of butanol as a solvent into a 250mL reaction flask, react under a 240W microwave for 30 seconds, take out and stir Mix well, then put it into the microwave for 15 seconds, take it out and stir well, repeat the last step 5 times. After the reaction was completed, it was filtered while it was hot, and the filtrate was rotary evaporated to remove and recover most of the solvent butanol, and dried in vacuum at 70°C for 48 hours to obtain the product trimethylbutyl quaternary ammonium hydrogen succinate ionic liquid, and the product yield was 96 %, chloride ion concentration 716ppm. Its chemical structural formula is:
[0023]
[0024] Characterization results: elemental analysis theoretical value C: 56.63%, H: 9.94%, N: 6.00%, measured value C: 56.65%, H: 9.91%, N: 6.0...
Embodiment 3
[0025] Embodiment 3: Trimethylbutyl quaternary ammonium hydrogen maleate ionic liquid
[0026]In 250mL reaction bottle, add trimethylbutyl ammonium chloride 15.12g (0.1mol) respectively, potassium hydrogen maleate or potassium hydrogen fumarate 15.42g (0.1mol) and solvent butanol 20g, in Under the microwave of 240W, react for 30 seconds, take it out and stir well, then put it into the microwave and react for 15 seconds, take it out and stir well, repeat the last step 4 times. After the reaction is completed, filter while it is hot, and rotate the filtrate to remove and recover most of the solvent butanol, and dry it in vacuum at 70°C for 48 hours to obtain the product trimethylbutyl quaternary ammonium hydrogen maleate ionic liquid or trimethylbutanol The base quaternary ammonium hydrogen fumarate ionic liquid has a product yield of 96% and a chloride ion concentration of 680ppm. Its chemical structural formula is:
[0027]
[0028] Characterization results: elemental ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com