Phosphazene flame retardant and preparation method thereof, as well as lithium ion battery electrolyte
A phosphazene-based flame retardant and lithium-ion battery technology, applied in the field of lithium-ion batteries, can solve problems such as poor system compatibility, affecting the electrochemical performance of lithium-ion batteries, and low conductivity of the electrolyte
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
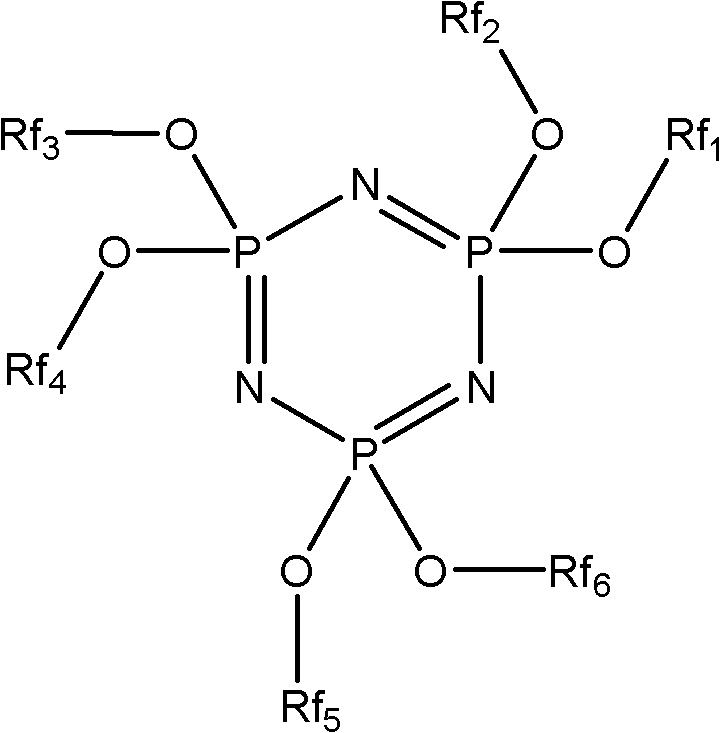
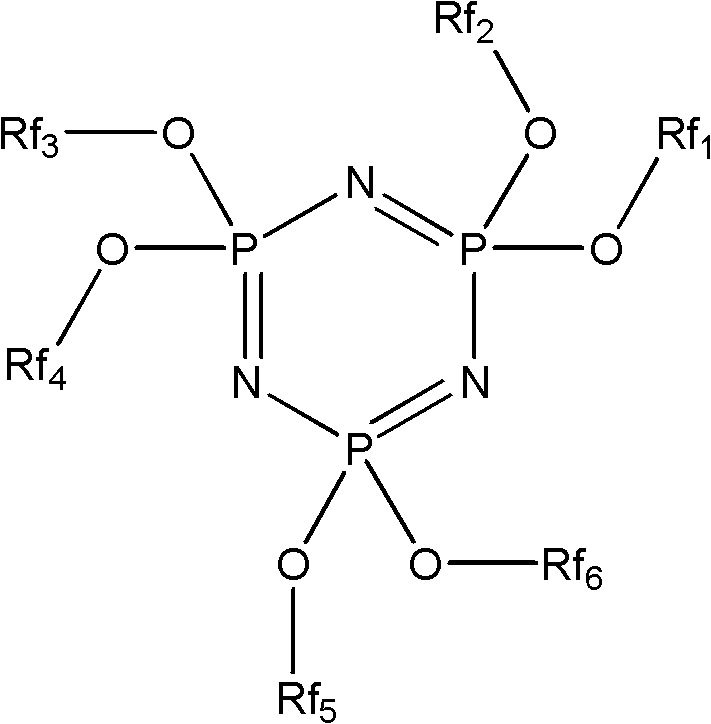
[0036] Correspondingly, the present invention also provides a preparation method of a phosphazene flame retardant, comprising the following steps:
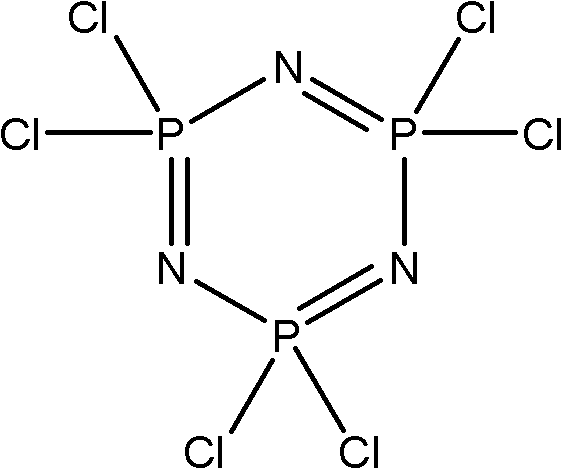
[0037] The fluorocarbon alcohol is reacted with hexachlorocyclotriphosphazene to obtain a phosphazene flame retardant, and the fluorocarbon alcohol is preferably a compound represented by formula II, formula III or formula IV. Wherein the fluorocarbon alcohol shown in formula II and formula III is obtained by tetrafluoroethylene telomerization (being commonly called as telomer alcohol), has been commercialized, as tetrafluoropropanol (TFP), octafluoropentanol (OFP) or DuPont's Zonyl Telomer BA series products; the compound represented by formula IV can be commercially available or can be prepared according to the method provided by the present invention.
[0038] H(CF 2 CF 2 ) n1 CH 2 Oh
[0039] Formula II
[0040] F(CF 2 CF 2 ) n2 CH 2 CH 2 Oh
[0041] Formula III
[0042] CF 3 CF 2 CF 2 O(CF(CF 3 ) CF 2 O) n3...
Embodiment 1
[0056] The structure of embodiment 1 fluorocarbon alcohol is H(CF 2 CF 2 ) n1 CH 2 OH, where 1≤n 1 ≤15.
[0057] Take 9.3g (0.165mol) KOH and 18.5ml deionized water into a three-necked flask, start stirring and heating, add dropwise a mixed solution of 0.165mol tetrafluoropropanol and 38.3ml xylene, the reaction solution changes from clear to milky white turbid, heat After 1 hour of reflux reaction, water was separated, and about 19ml of water was separated, and the reaction solution turned yellowish brown. A mixed solution of 8.7g (0.025mol) trimeric phosphazene and 60ml xylene was added dropwise, and the reaction was carried out under reflux for 50h. Pour the reaction solution obtained above into a beaker, wash with water until neutral, add 20g of anhydrous Na 2 SO 4Drying, suction filtration, and concentration under reduced pressure gave 21.88 g of a reddish-brown liquid crude product, which was then purified by high vacuum distillation (0.5 mmHg) to obtain 16.37 g of...
Embodiment 2
[0058] The structure of embodiment 2 fluorocarbon alcohol is F(CF 2 CF 2 ) n2 CH 2 CH 2 OH, where n 2 =1≤n 2 ≤15.
[0059] Take 9.3g (0.165mol) KOH and 18.5ml deionized water into a three-necked flask, start stirring, heat, drop 0.165mol F(CF 2 CF 2 ) n2 CH 2 CH 2 OH(n 2 =3) and 60ml xylene mixed solution, the reaction solution turns from clear to milky white turbidity, heated to reflux reaction for 1h and then separated water, about 19ml of water separated reaction solution turned yellowish brown, and 8.7g (0.025mol) trimeric solution was added dropwise The mixed solution of phosphazene and 60ml xylene was refluxed for 55h under reflux. Pour the reaction solution obtained above into a beaker, wash with water until neutral, add 20g of anhydrous Na 2 SO 4 Drying, suction filtration, concentration under reduced pressure to obtain 48.8g of reddish-brown liquid crude product; then carry out high vacuum distillation purification (0.2mmHg) to obtain colorless transpare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com