Preparation method of compound and its application
A technology for compounds and microbial strains, applied in the preparation of compounds and their application fields, can solve problems such as high cost and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
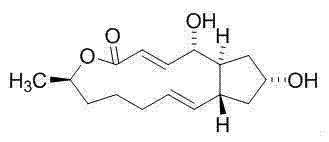
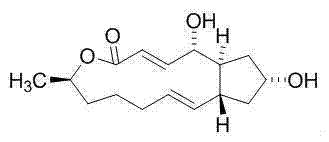
[0027] Compound 1,13-dihydroxy-6-methyl-6,7,8,9,12,13,14,14a-octanehydro-1H-cyclopentane[ f ][1]Separation and preparation of epoxytridecane-4(11aH)-one
[0028] a. The fungus Phomopsis ( Phomopsis chimonanthi ) activation:
[0029] PDA medium: 200 g of potato, 20 g of glucose, 15 g of agar, 1000 mL of water, make a test tube slope, pick mycelium and inoculate it on the test tube slope, 25 o C was cultured for 7 days; the concentration of spores obtained was 1 × 10 6 Individual / mL bacterial suspension;
[0030] b. The fungus Phomopsis ( Phomopsis chimonanthi ) solid fermentation culture:
[0031] Rice medium (preparation process: add 100 g rice and 100 mL water into a 500 mL Erlenmeyer flask, soak overnight, sterilize and cool for later use).
[0032] The prepared bacterial suspension (spore concentration of 1 × 10 6 individual / mL) 5 mL was inoculated on the rice medium, 25 o C cultured for 40 days;
[0033] c. After the fermentation is complete, add ethyl acetate i...
Embodiment 2
[0038] The test of the anti-Aspergillus fumigatus activity of the compound of the present invention is detected by fungi 96 empty microwell plate method antibacterial method
[0039] 1 Preparation of bacterial suspension
[0040] fungus Aspergillus fumigatus Activated on PDB medium for 48 h, so that the bacteria are in the logarithmic growth phase. Take 1–2 single colonies and add them to the corresponding liquid medium, adjust the bacterial concentration so that the bacterial concentration of the fungus is 0.5–2.5×10 4 cfu / mL.
[0041] 2 Sample preparation
[0042] Dissolve the sample with DMSO or water as solvent. If DMSO is used as the solvent, since high concentrations of DMSO may have an inhibitory effect on the test bacteria, it should be diluted with water (generally, the concentration of DMSO should be controlled within 5%).
[0043] 3 add sample
[0044]Take a clean and sterile 96-well cell culture plate. Generally, column A is used as a blank control, and 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com