Cyclosporine-containing ophthalmic emulsion gel and preparation method thereof
A technology of cyclosporine and emulsion, which is applied in the field of medicine, can solve the problems of patients' intolerance, high permeability, eye irritation, etc., and achieve the goal of reducing gastrointestinal irritation, fully absorbing and utilizing it, and reducing other side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
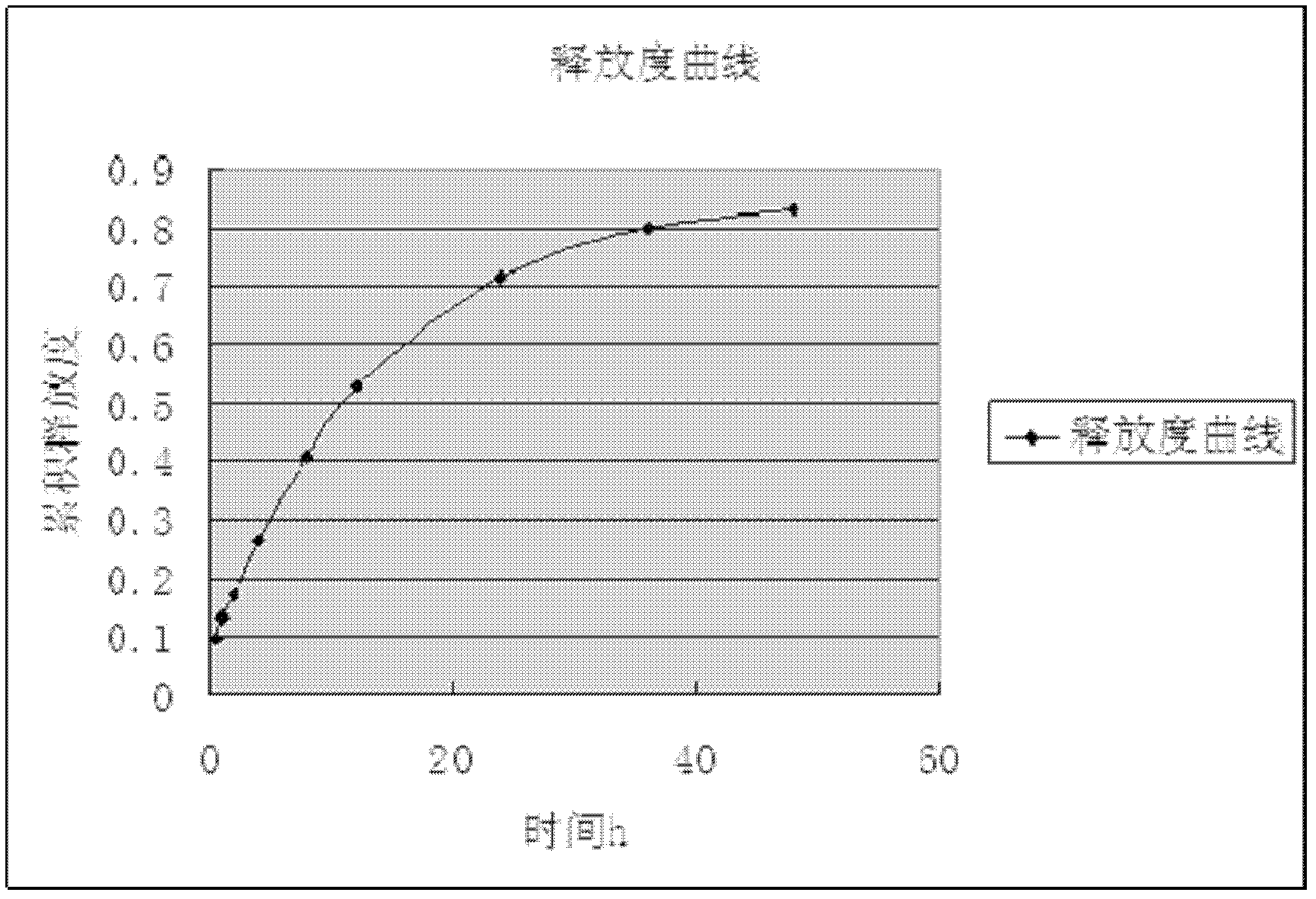
Image
Examples
Embodiment 1
[0032] Ophthalmic emulsion gel containing cyclosporine, the formula is as follows: cyclosporine A 0.2%, peanut oil 2%, carbomer 5%, sodium chloride 0.1%, Tween 80 5%, NAOH appropriate amount, add water for injection into 150g ophthalmic emulsion gel containing cyclosporine.
[0033] The steps are:
[0034] (1) Preparation of emulsion:
[0035] a. Preparation of oil phase: Precisely weigh cyclosporine A into a 10mL eggplant-shaped bottle, add peanut oil to dissolve cyclosporine A, then add pH regulator sodium hydroxide to adjust pH to 7, and obtain the oil phase;
[0036] b. Preparation of the water phase: accurately weigh the prescription amount of Tween 80 to 1 part of water for injection to obtain the water phase;
[0037] c. Mixing: Weigh the oil phase according to the ratio of oil phase: water phase to 1:5 and add it to the water phase to obtain the emulsion after mixing;
[0038] (2) Swelling of the gel matrix: Take 10 parts of water for injection into a beaker, spread...
Embodiment 2
[0041] Ophthalmic emulsion gel containing cyclosporine, the formula is as follows: cyclosporine A 0.4%, dimethyl sulfoxide 3%, glyceryl monostearate 0.3%, sodium hyaluronate 5%; mannitol 4%; hydrogen Appropriate amount of sodium oxide, add water for injection to make 150g ophthalmic emulsion gel containing cyclosporine.
[0042] The steps are:
[0043] (1) Preparation of emulsion:
[0044] a. Preparation of oil phase: Accurately weigh cyclosporine A into a 10mL eggplant-shaped bottle, add dimethyl sulfoxide to dissolve cyclosporine A, then add a pH regulator to adjust the pH to 7, and then obtain the oil phase;
[0045] b. Preparation of the water phase: Accurately weigh glycerol monostearate into 1 part of water for injection to obtain the water phase;
[0046] c. Mixing: Weigh the oil phase according to the ratio of oil phase: water phase of 1:15 and add it to the water phase, and then get the emulsion after mixing;
[0047] (2) Swelling of the gel matrix: Take 10 parts o...
Embodiment 3
[0050] Ophthalmic emulsion gel containing cyclosporine, the formula is as follows: cyclosporine A 0.1%, soybean oil 3%, polyvinyl alcohol 0.3%, hydroxymethylcellulose sodium 3%; glycerin 3%; sodium hydroxide appropriate amount, Add water for injection to make 150 g ophthalmic emulsion gel containing cyclosporine.
[0051] The steps are:
[0052](1) Preparation of emulsion:
[0053] a. Preparation of oil phase: Accurately weigh cyclosporine A into a 10mL eggplant-shaped bottle, add soybean oil to dissolve cyclosporine A, then add a pH regulator to adjust the pH to 7, and then obtain the oil phase;
[0054] b. Preparation of the water phase: Precisely weigh polyvinyl alcohol into 1 part of water for injection to obtain the water phase;
[0055] c. Mixing: Weigh the oil phase according to the ratio of oil phase: water phase of 1:20 and add it to the water phase to obtain the emulsion after mixing;
[0056] (2) Swelling of the gel matrix: Take 10 parts of water for injection in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com