Preparation method of bisphenol A epoxy ethane additive product
A technology of ethylene oxide and adduct, which is applied in the field of preparation of bisphenol A ethylene oxide adduct, can solve the problems of product performance influence, residue, etc., and achieves a reaction with good reaction selectivity and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
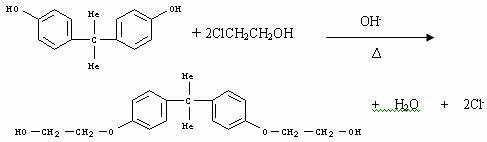
[0030] The steps of the preparation method of bisphenol A ethylene oxide adduct are as follows:
[0031] (1) Dissolve 1 mole of bisphenol A and 0.001~0.01 mole of catalyst in 2~10 moles of ether solvent at 80~150°C, and add the solution in 5~10 moles of n-hexane or n-heptane dropwise at this temperature 2.05~2.30 moles of ethylene oxide in the solution, keep it warm until the residual bisphenol A is less than 10ppm;
[0032] (2) Slowly lower the temperature to 0-30°C for crystallization, then filter, wash with n-hexane or n-heptane, and vacuum-dry to obtain a product with a content of more than 98% of bisphenol A plus 2 moles of ethylene oxide;
[0033] (3) The crystallization mother liquor is rectified under normal pressure or vacuum to separate n-hexane or n-heptane, which can be used together with the contained catalyst in the next reaction process.
[0034] The catalyst refers to tri-n-butylamine, tri-n-octylamine or tri-n-decylamine.
[0035]The ether solvent is di-n-pr...
Embodiment 1
[0038] Add 228g (1 mole) bisphenol A, 0.101g (0.001 mole) tri-n-butylamine catalyst and 436g (2 moles) diethylene glycol di-n-butyl ether in a 3L pressure-resistant glass bottle with stirring, and heat up to 150 ℃ to dissolve into a transparent solution; dissolve 90.2g (2.05 moles) of ethylene oxide in 1000g (10 moles) of n-heptane; add the above ethylene oxide dropwise through a constant pressure dropping funnel within 4 hours at a temperature of 150°C The n-heptane solution of alkane, after the dropwise addition, continue the reaction until the residual bisphenol A detected by HPLC is less than 10ppm. After the reaction is completed, slowly lower the temperature to 30°C and keep it warm for 5 hours to fully crystallize, then filter with suction, wash the crystals with 300g of n-heptane, and dry in vacuum at 80°C to constant weight to obtain 301.8g of BHE-BPA finished product, the HPLC detection content is 98.7 %.
Embodiment 2
[0040] The mother liquor obtained in Example 1 was rectified under reduced pressure in a 3000ml three-neck flask with a 50cm rectifying column to obtain 1280g of reclaimed n-heptane and 451g of reclaimed diethylene glycol di-n-butyl ether containing catalyst tri-n-butylamine. Add 228g (1 mole) of bisphenol A and 451g of diethylene glycol di-n-butyl ether recovered above into a 3L pressure-resistant glass bottle with stirring, and dissolve it at 150°C to become a transparent solution; Dissolve ethylene oxide in 1000g (10 moles) of the recovered n-heptane; add the above-mentioned n-heptane solution of ethylene oxide dropwise within 4 hours at a temperature of 150°C, and continue the reaction until bisphenol A is detected by HPLC The residue is less than 10ppm. After the reaction is completed, slowly lower the temperature to 30°C and keep it warm for 5 hours to fully crystallize, then filter with suction, wash the crystals with 300g of n-heptane, and dry in vacuum at 80°C to cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com