Method for synthetizing DL-cysteine
A synthesis method and cysteine technology are applied in the synthesis field of DL-cysteine, can solve the problems of high production cost, complicated process, low yield and the like, and achieve low production cost, high reaction yield, and high production efficiency. simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
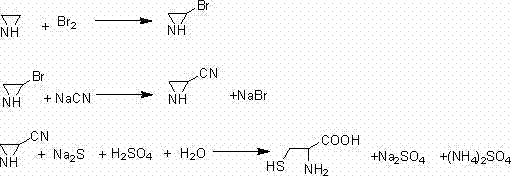
[0021] A synthetic method for DL-cysteine, comprising the steps of:
[0022] (1) Using ethyleneimine to react with bromine water to obtain 2-bromo-ethyleneimine;
[0023] (2) adding cyanide to the 2-bromo-ethyleneimine prepared in step (1) for heat preservation reaction to obtain 2-cyano-ethyleneimine;
[0024] (3) ring-opening reaction of 2-cyano-ethyleneimine prepared in step (2) with sodium sulfide to generate 2-mercapto-3-aminopropylcyanide;
[0025] (4) Acid hydrolysis of 2-mercapto-3-aminopropanil produced in step (3) at 60°C-90°C to obtain DL-cysteine.
[0026] Wherein, in step (1), the molar ratio of ethyleneimine to bromine water is 1:0.8-1.1; the reaction temperature is 0°C-40°C, and the reaction time is 1-6h; the The preferred reaction temperature is 5°C-30°C, and the preferred reaction time is 2-5h.
[0027] In step (2), the molar ratio of 2-bromo-ethyleneimine to sodium cyanide is 1:1-1.1; the reaction temperature is 0°C-80°C, and the reaction time is 2 -5h; t...
Embodiment 1
[0032] Add 43.4g of ethyleneimine into a 500ml flask, control the temperature at 40°C, add 129.3g of bromine water under stirring, and keep the reaction for 4h. Add 50g of sodium cyanide to the flask, keep it warm for 5 hours, control the temperature to 50°C, add 200ml of acetone, continue stirring for 1 hour, remove the sodium bromide by filtration, and use the filtrate for later use. Put 78 g of sodium sulfide and 150 ml of water into a 1000 ml flask, raise the temperature to 55°C, then add the above filtrate dropwise into the sodium sulfide aqueous solution, after the dropwise addition, keep warm for 2 hours, then add 200g of 98% sulfuric acid dropwise. Heat up to 80°C, heat-preserve and hydrolyze for 4 hours, filter to remove inorganic salts, cool the filtrate to 5°C, filter, wash the filter cake with 100ml of acetone, and dry to obtain 83.5g of DL-cysteine with a yield of 68.39%.
Embodiment 2
[0034] Add 43.4g of ethyleneimine into a 500ml flask, control the temperature at 0°C, add 177.8g of bromine water under stirring, and keep the reaction for 1h. Add 55g of sodium cyanide to the flask, keep it warm for 4 hours, control the temperature to 38°C, add 200ml of acetone, continue stirring for 1 hour, remove the sodium bromide by filtration, and use the filtrate for later use. Put 82g of 98% sodium sulfide and 150ml of water into a 1000ml flask, raise the temperature to 60°C, then add the above-mentioned filtrate dropwise into the sodium sulfide aqueous solution, after the dropwise addition, keep warm for 3 hours, then add 200g of 98% sulfuric acid dropwise. Heat up to 80°C, heat-preserve and hydrolyze for 4 hours, filter to remove inorganic salts, cool the filtrate to 5°C, filter, wash the filter cake with 100ml acetone, and dry to obtain DL-cysteine 77g, yield 63.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com