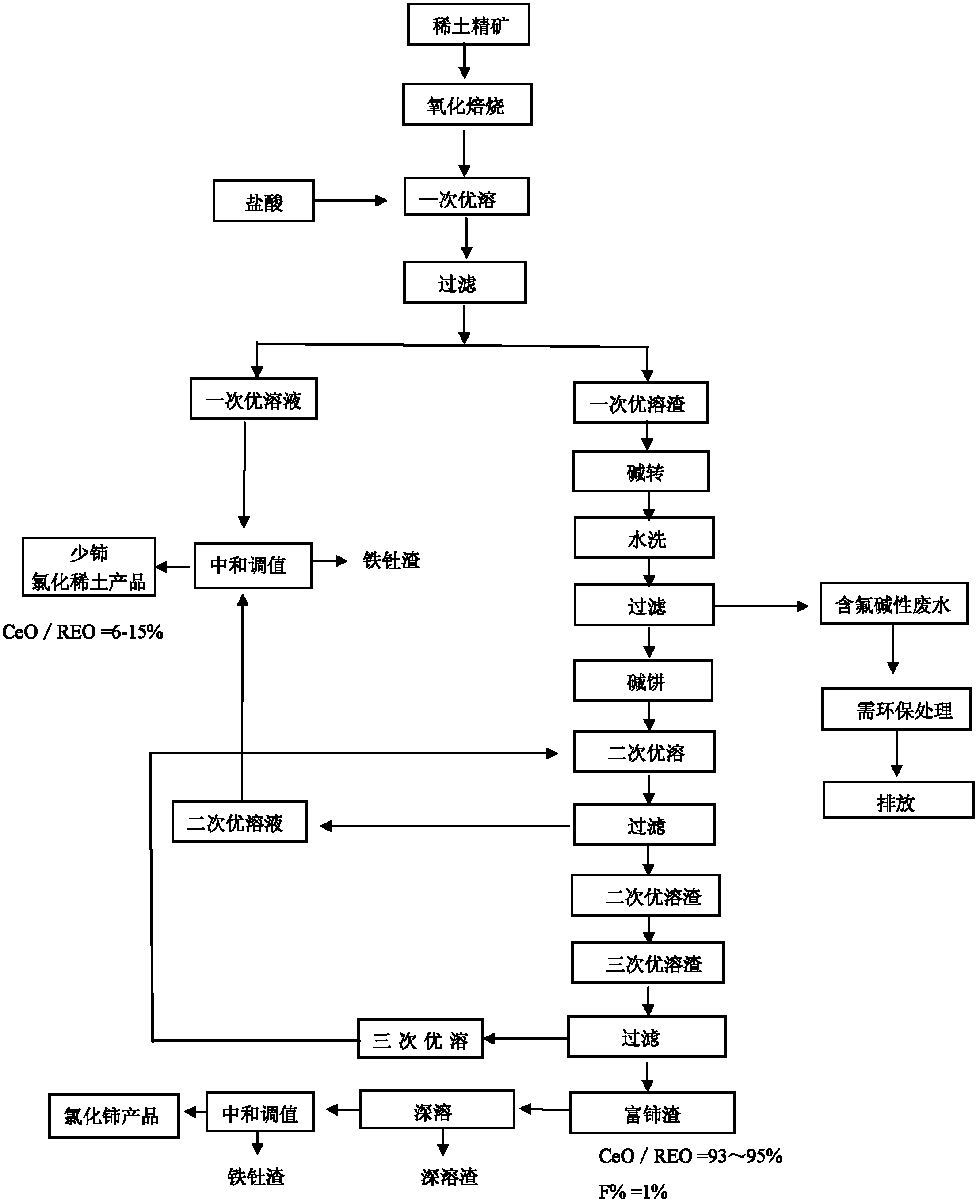
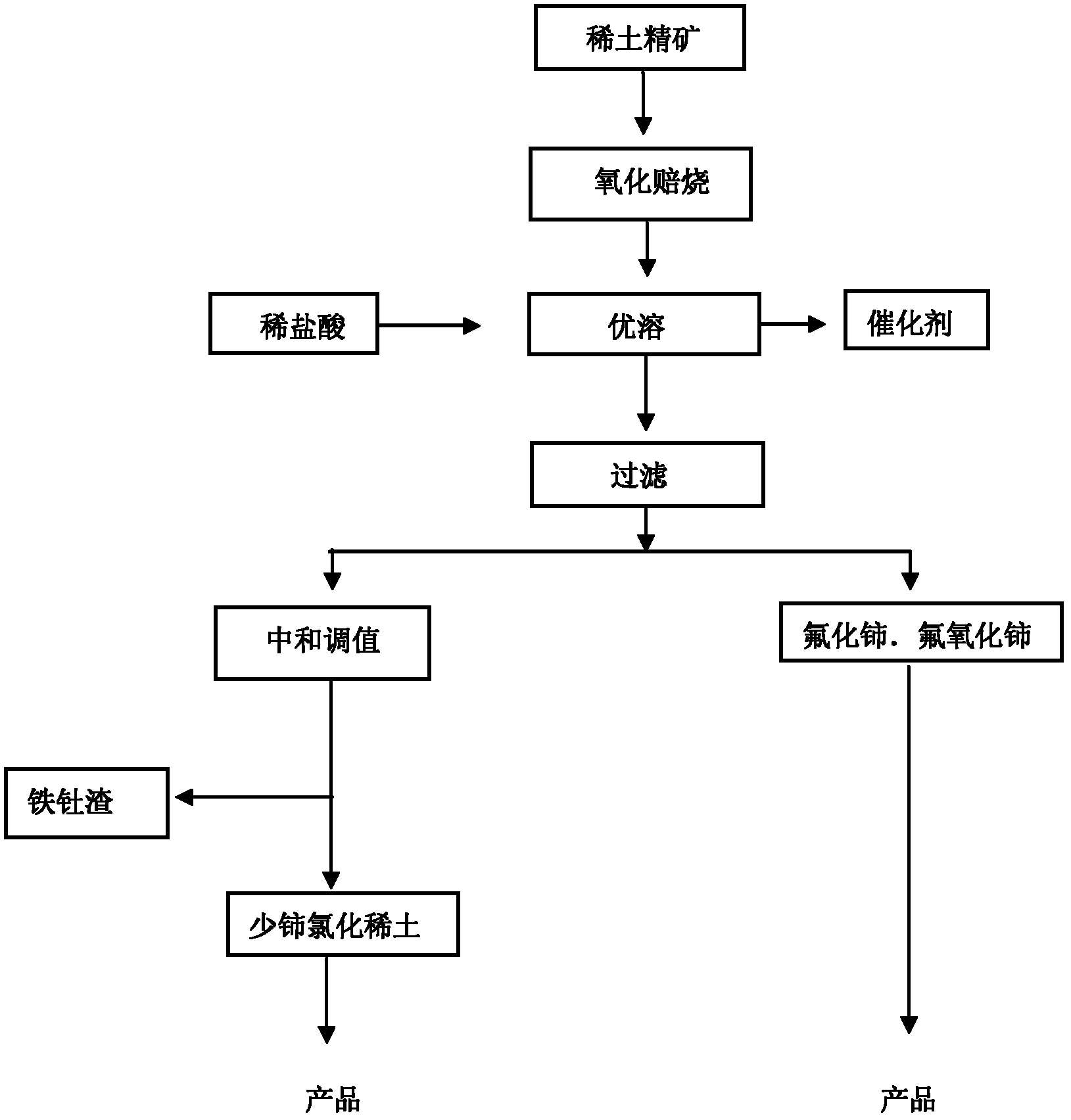
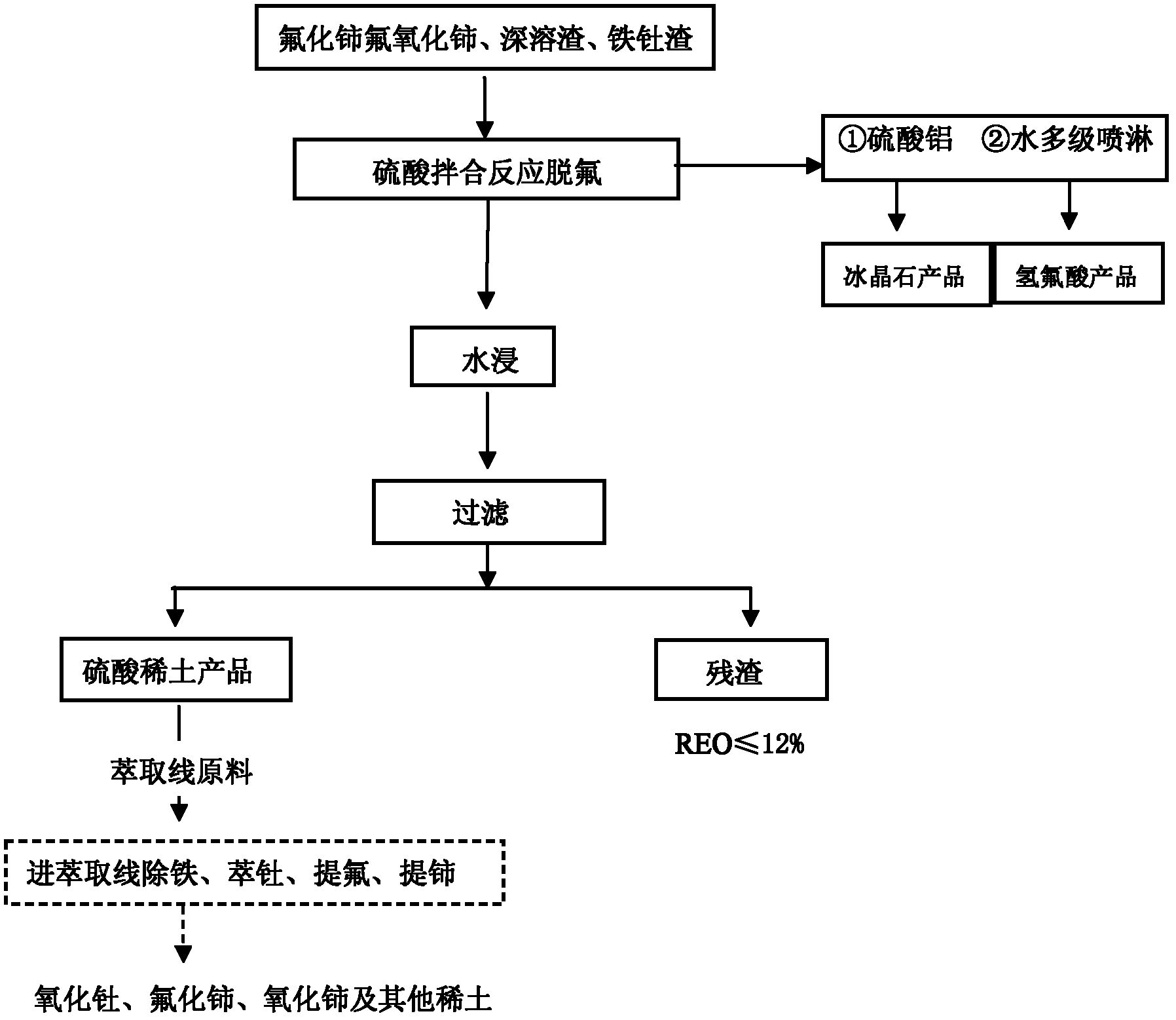
Method for comprehensively recycling various rare earth from rare earth materials containing fluorine
A rare earth and material technology, applied in the field of non-ferrous metal rare earth separation, can solve the problems of high treatment cost, rare earth loss, high labor intensity, etc., and achieve the effect of solving the problems of radioactive thorium pollution and fluorine pollution, solving environmental protection problems, and diversifying products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Two samples of deep molten slag (number ①, ②)
[0080] Raw materials: deep molten slag 200g (containing about 10% fluorine, REO 55.1%, that is, containing 110.2 grams of rare earth oxides);
[0081] ①(200*55.1%)=110.2 grams of rare earth
[0082] ②(200*55.1%)=110.2 grams of rare earth
[0083] 98% industrial sulfuric acid;
[0084] Acid addition ratio: weight ratio REO: sulfuric acid = 1: 1.55, 170.81 ml sulfuric acid
[0085] Two samples of fluorine-containing and rare earth-containing deep molten slag (number ①, ②) are mixed (stirred) with 98% sulfuric acid. After the mixing reaction, the slag is semi-dry, and the HF gas formed by the violent reaction is carried out by water. Multi-stage spray absorption to obtain hydrofluoric acid solution. 800 milliliters of the mixed material was added, and the rare earth sulfate was obtained by water immersion. The water immersion reaction of No. 1 sample was 2 hours, and the No. 2 sample was 2.5 hours.
[0086] Res...
Embodiment 2
[0090] Two samples of embodiment 2 cerium fluoride (table number 3., 4.)
[0091] Raw materials: 200 grams of cerium fluoride (62.7% rare earth content, 14% fluorine content)
[0092] ③(200*62.7%)=125.3 grams of rare earth
[0093] ④(200*62.7%)=125.3 grams of rare earth
[0094] Industrial sulfuric acid (98%);
[0095] Acid addition ratio: weight ratio REO: sulfuric acid = 1: 1.55, 170.81 ml sulfuric acid
[0096] Mix (stir) two samples of fluorine-containing and rare earth-containing deep molten slag (number ③, ④) with 98% sulfuric acid. After the mixing reaction, the slag is semi-dry, and the HF gas formed by the violent reaction is carried out by water. Multi-stage spray absorption to obtain hydrofluoric acid solution. 800 milliliters of the mixed material was added, and the rare earth sulfate was obtained by water immersion. The ③ sample was immersed in water for 2 hours, and the ④ sample was 2.5 hours.
[0097] Residue weighing:
[0098] ③ is 75 grams, wherein the r...
Embodiment 3
[0101] A sample of embodiment 3 iron thorium slag (table number ⑤)
[0102] Raw materials: 300 grams of iron thorium slag (including 24% rare earth content and 3% fluorine content)
[0103] ⑤(300*24%)=72 grams of rare earth
[0104]Industrial sulfuric acid (98%);
[0105] Acid addition ratio: weight ratio REO: sulfuric acid=1: 2, 144 milliliters of sulfuric acid;
[0106] Mix (stir) two samples of deep molten slag containing fluorine and rare earth materials (number ⑤) with 98% sulfuric acid. After the mixing reaction, the slag is semi-dry, and the HF gas formed by the violent reaction passes through 0.1M aluminum sulfate The solution is multi-stage sprayed and absorbed to obtain aluminum fluoride solution Al 3+ The concentration is about 0.1mol / L, adjust the pH value of the solution to 4.5-5.5, add sodium salt to it according to the F / Al ratio of 5.5-6, and the Na / Al ratio of 3.0-3.1, the reaction temperature is above 90°C, and the reaction time is For 60min, cryolite was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com