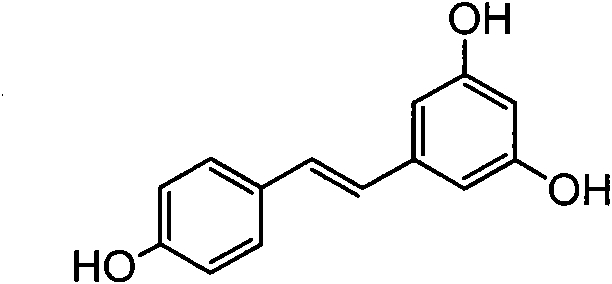
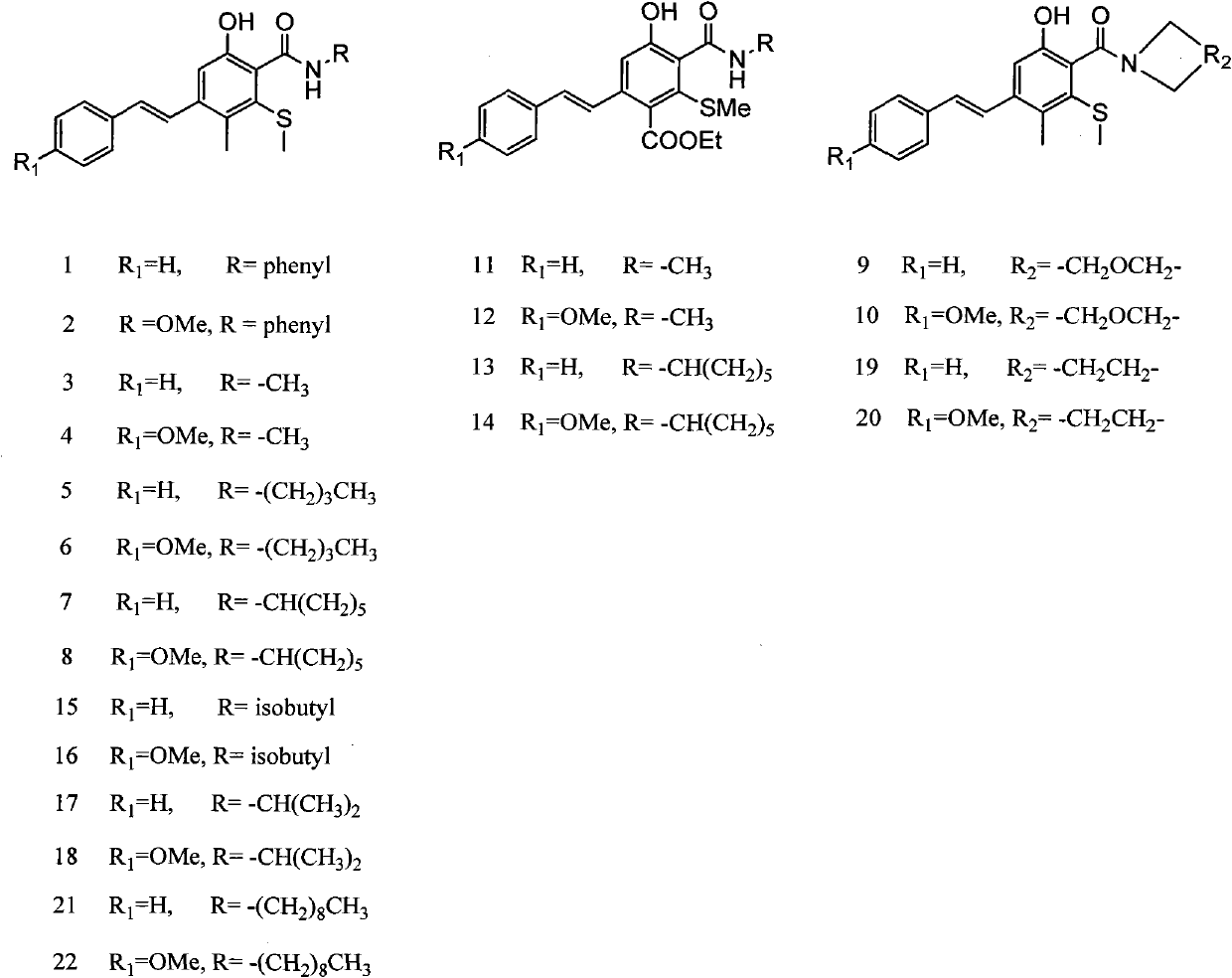
Method for synthesizing anti-form stilbene compounds and application of method in preparing anti-tumor medicines
A technology of stilbene compounds and anti-tumor drugs, which is applied in the field of synthesizing trans-stilbene compounds, can solve the problems of reduced yield, difficult separation, and difficulty in obtaining compounds, and achieves the effects of simple method, reduced cost, and increased difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
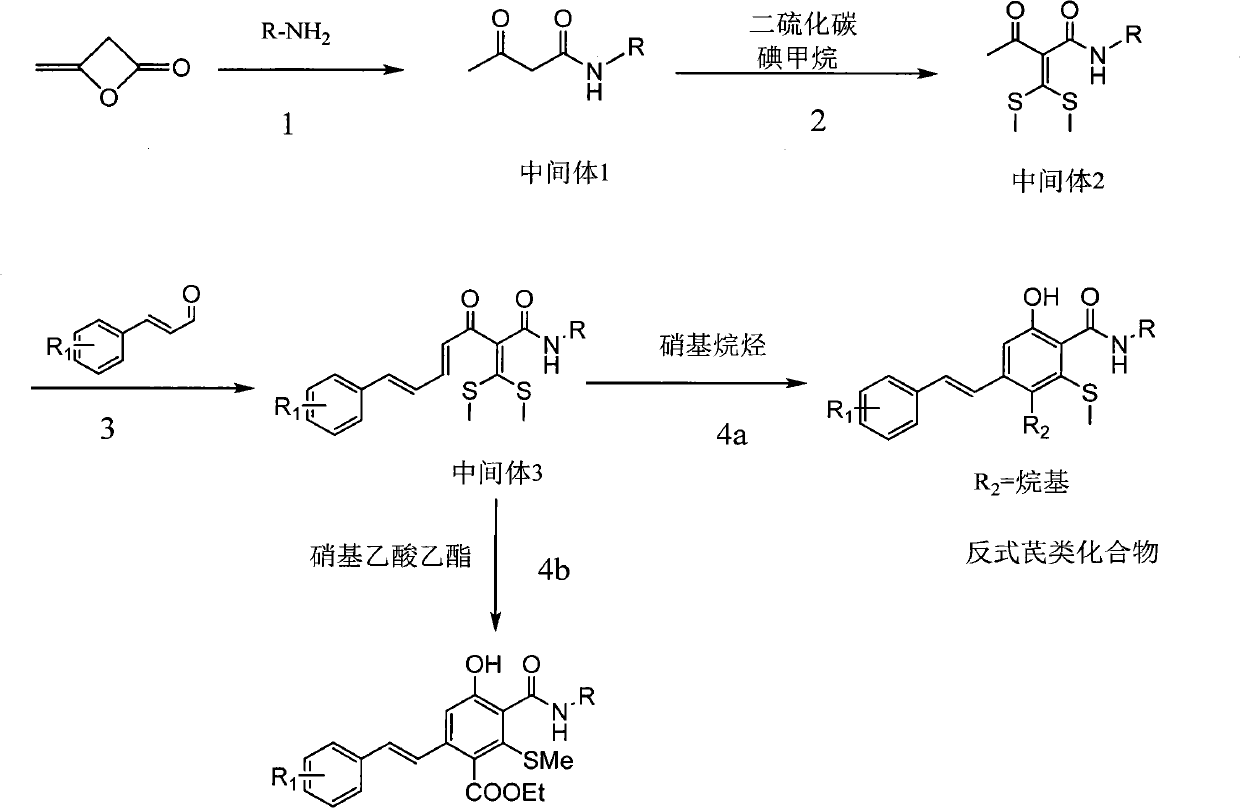
[0033](1) Preparation of acetoacetylnonylamine: Dissolve 20mmol of nonylamine in 50ml of water, cool down to 0°C, add 20mmol of diketene dropwise within 1 hour, react for 2 hours after dropping, extract with dichloromethane, wash the organic layer with water, and dry , filtered with suction, concentrated to dryness under reduced pressure to obtain acetoacetylnonylamine with a yield of 96%;
[0034] (2) Preparation of 2-(bis(methylthio)methenyl)-N-nonyl-3-oxobutanamide: 20mmol of acetoacetylnonylamine obtained in the previous step was dissolved in 50ml of N,N-dimethyl In base formamide, cool down to 0°C, add 50mmol potassium carbonate, stir at 0°C for 30min, then add 24mmol carbon disulfide, react at 0°C for 1 hour, then add 40mmol methyl iodide dropwise within 30min, rise to React at 10-35°C for 4 hours, pour into water, a yellow solid precipitates out, filter with suction, wash with water, and dry to obtain 2-(di(methylthio)methenyl)-N-nonyl-3-oxobutyramide , yield 88%;
[...
Embodiment 2
[0038] (1) Preparation of acetoacetylnonylamine: Dissolve 20mmol of nonylamine in 50ml of benzene, cool to 0°C, add 20mmol of diketene dropwise within 1 hour, react for 2 hours after dropping, wash the organic layer with water, dry, and suction filter. Concentrated to dryness under reduced pressure to obtain acetoacetylnonylamine with a yield of 93%;
[0039] (2) Preparation of 2-(bis(methylthio)methenyl)-N-nonyl-3-oxobutanamide: 20mmol of acetoacetylnonylamine obtained in the previous step was dissolved in 50ml of N,N-dimethyl In base formamide, cool down to 0°C, add 50mmol potassium carbonate, stir at 0°C for 30min, then add 24mmol carbon disulfide, react at 0°C for 1 hour, then add 40mmol methyl iodide dropwise within 30min, rise to 10 React at ~35°C for 4 hours, pour into water, a yellow solid precipitates, filter with suction, wash with water, and dry to obtain 2-(di(methylthio)methenyl)-N-nonyl-3-oxobutyramide, Yield 88%;
[0040] (3) (4E,6E)-2-(di(methylthio)methenyl)...
Embodiment 3
[0043] (1) Preparation of acetoacetylmorpholine: Dissolve 20mmol morpholine in 50ml benzene, cool to 0°C, add 20mmol diketene dropwise within 1 hour, react for 2 hours after dropping, wash the organic layer with water, dry, and suction filter, Concentrated to dryness under reduced pressure to obtain acetylacetylmorpholine with a yield of 89%;
[0044] (2) Preparation of 2-(bis(methylthio)methenyl)-1-morpholinyl-1,3-butanedione: 20mmol of acetylacetylmorpholine obtained in the previous step was dissolved in 50ml N,N- In dimethylformamide, cool down to 0°C, add 50mmol potassium carbonate, stir at 0°C for 30min, then add 24mmol carbon disulfide, react at 0°C for 1 hour, then add 40mmol methyl iodide dropwise at about 30min, after the addition is complete Rise to 10-35°C for 4 hours, remove N,N-dimethylformamide, extract with dichloromethane, wash with water, dry, filter with suction, concentrate under reduced pressure to dryness, and obtain 2-(bis(methylthio)methylene Base)-1-mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com