A blood replacement base fluid and its preparation method
A technology of blood replacement and base fluid, which is applied in the fields of blood diseases, medical formulas, and extracellular fluid diseases, etc. It can solve the problems of reducing the anticoagulant effect of replacement base fluid, unstable blood calcium ion concentration, and physiological dysfunction of patients, etc., to achieve The effect of preventing hypocalcemia, good anticoagulant effect, and saving clinical operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of blood replacement base fluid of the present invention
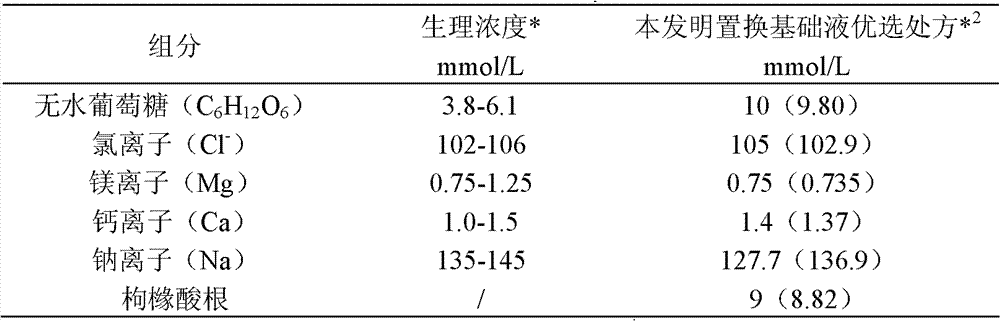
[0036] Take 10 moles of anhydrous glucose, 100.7 moles of sodium chloride, 0.75 moles of magnesium chloride hexahydrate, 1.4 moles of calcium chloride dihydrate, and 9 moles of sodium citrate dihydrate, dissolve them in 400 liters of water for injection, and then add activated carbon for adsorption. Filter with a 0.45 μm titanium rod, add water for injection to 1000 liters, filter through a 0.22 μm terminal filter, fill it into a PVC infusion bag with a capacity of 4000 ml, seal it, and sterilize at 115 degrees Celsius for 30 minutes to obtain the product.
[0037] In the prepared replacement base solution, the concentration of each component is shown in Table 1:
[0038] Table 1
[0039]
[0040] Note *: The physiological concentration here refers to the concentration of free electrolytes in the extracellular fluid components (mainly serum) of the human physiological body.
[0041]...
Embodiment 2
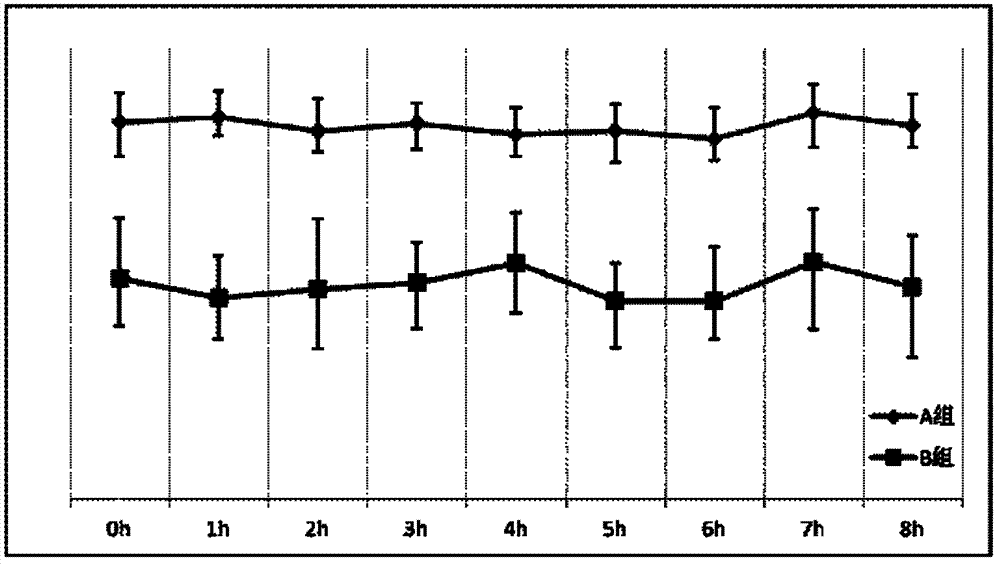
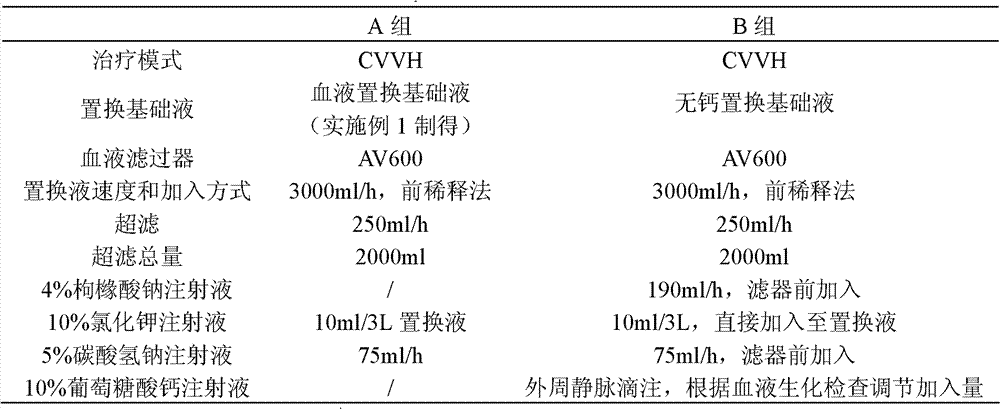
[0042] Embodiment 2 The screening of the concentration of each component in the blood replacement base fluid of the present invention
[0043] The principle of citrate anticoagulation is that citrate complexes free calcium ions in the blood at 1:1 to block the blood coagulation chain. Or the content of sodium citrate should be at least greater than the sum of the calcium content in the replacement base fluid and the amount of free calcium ions in the mixed blood during use. Application in replacement therapy, China Blood Purification, 2005, 4(1): 42], the amount of replacement base fluid is mostly 2000-4000 ml / hour, and the blood flow is mostly 120-160 ml / min. Based on this calculation, the replacement base The citrate contained in the solution should at least be able to complex and replace the calcium content in the base solution (0.225 mmol / L), and the amount of free calcium ions in the corresponding blood (the highest is: the upper limit of the concentration of free calcium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com