Compound tetracaine composition as well as preparation method and application thereof
A technology of compound Dingka and composition, which is applied in the direction of drug combination, effective ingredients of hydroxyl compounds, and pharmaceutical formulas, etc., to achieve the effects of convenient use, promotion of wound healing, and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
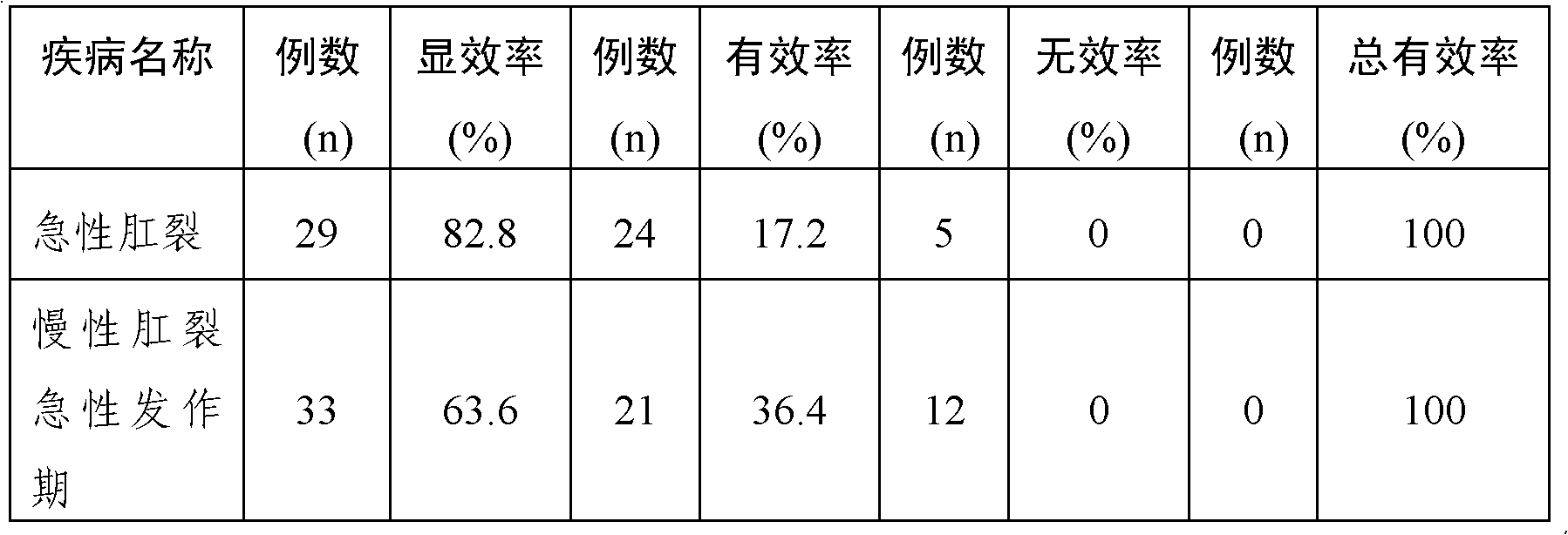
Embodiment 1
[0039] Embodiment 1: ointment
[0040] 1. Prescription: In each g ointment, tetracaine hydrochloride 3.0mg, ephedrine hydrochloride 3.1mg, phenobarbital 3.0mg, diphenhydramine hydrochloride 1.6mg, hydrocortisone 0.7mg, borneol 8.0mg, mint oil 3ml (density: 0.888-0.908g / ml), add yellow petrolatum to 1000g, and prepare 1000g ointment. The ointment prepared in this example has the best anti-inflammatory and analgesic effects.
[0041] 2. Preparation method: After mixing the coarse powder raw materials of tetracaine hydrochloride, ephedrine hydrochloride, phenobarbital, diphenhydramine hydrochloride, hydrocortisone, and borneol according to the method of increasing the dosage in this embodiment , dried and pulverized into fine powder for later use; melt yellow vaseline at 50° C., and add it to the pulverized fine powder according to this embodiment. Add peppermint oil during stirring, and mix until semi-solidified.
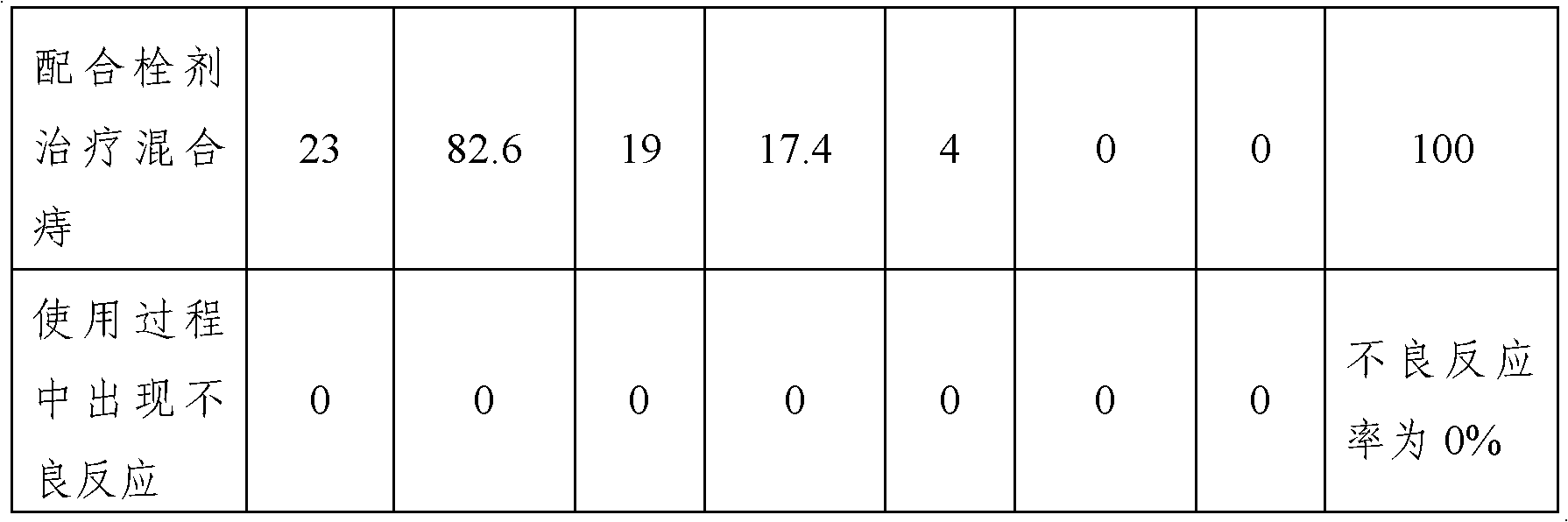
Embodiment 2
[0042] Embodiment 2: ointment
[0043] 1. Prescription: In each g ointment, tetracaine hydrochloride 3.3mg, ephedrine hydrochloride 2.8mg, phenobarbital 3.3mg, diphenhydramine hydrochloride 1.4mg, hydrocortisone 0.9mg, borneol 6.0mg, mint oil 3.5ml (density: 0.888-0.908g / ml), add white petrolatum to 1000g, and prepare 1000g ointment.
[0044] 2. Preparation method: After mixing the coarse powder raw materials of tetracaine hydrochloride, ephedrine hydrochloride, phenobarbital, diphenhydramine hydrochloride, hydrocortisone, and borneol according to the method of increasing the dosage in this embodiment , dried and pulverized into fine powder for later use; melt white vaseline at 60° C., and add it to the above pulverized fine powder according to the proportion of this embodiment. Add peppermint oil during stirring, and mix until semi-solidified.
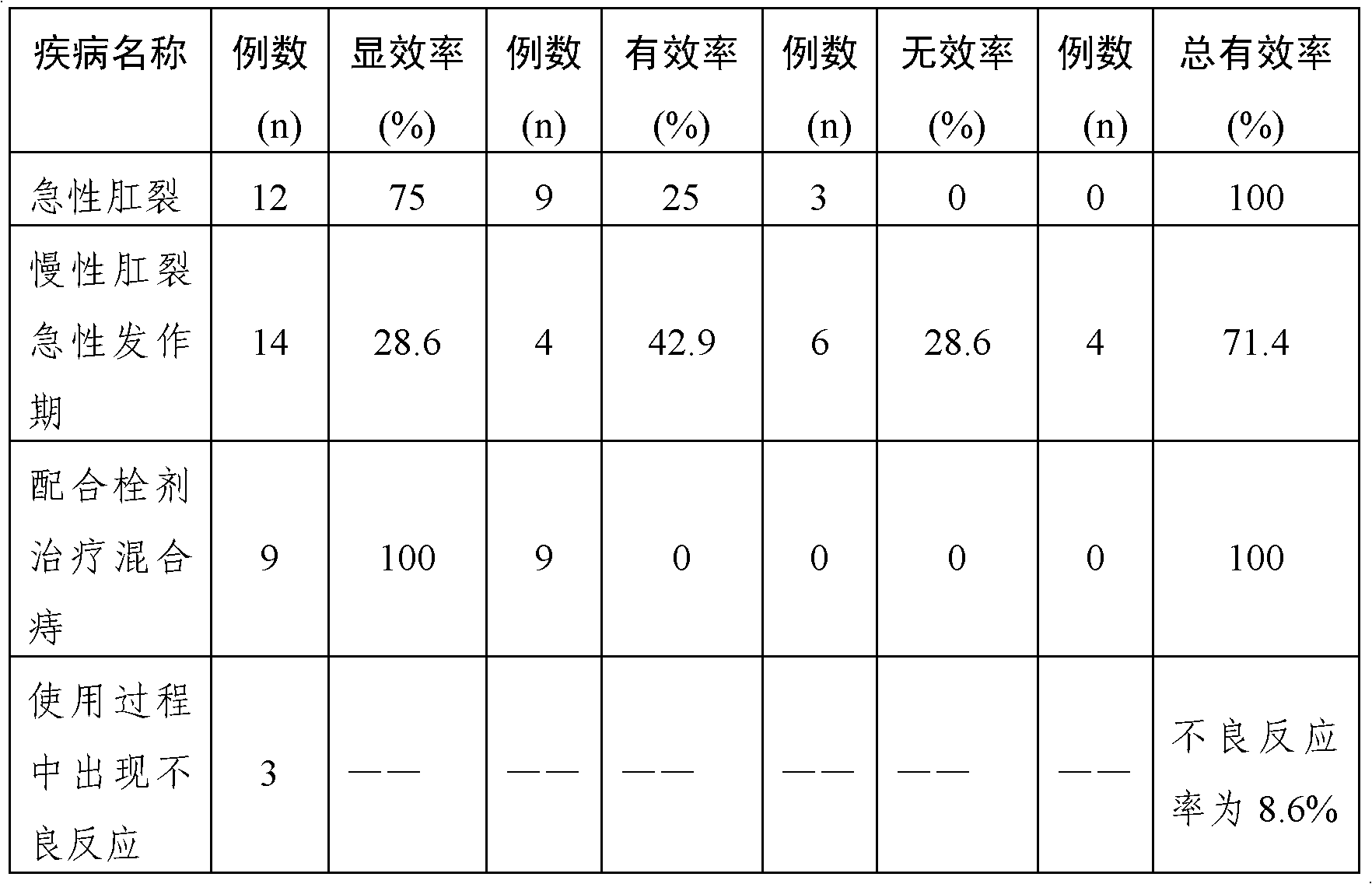
Embodiment 3
[0045] Embodiment 3: ointment
[0046] 1. Prescription: In each g ointment, tetracaine hydrochloride 2.7mg, ephedrine hydrochloride 3.4mg, phenobarbital 2.7mg, diphenhydramine hydrochloride 1.8mg, hydrocortisone 0.5mg, borneol 1.0mg, mint oil 3.2ml (density: 0.888-0.908g / ml), add yellow petrolatum to 1000g, and prepare 1000g ointment.
[0047] 2. Preparation method: After mixing the coarse powder raw materials of tetracaine hydrochloride, ephedrine hydrochloride, phenobarbital, diphenhydramine hydrochloride, hydrocortisone, and borneol according to the method of increasing the dosage in this embodiment , dried and pulverized into fine powder for later use; melt yellow vaseline at 55° C., and add it to the pulverized fine powder according to this embodiment, and adjust the mixer at a speed of 200 for uniform stirring in the molten state Add peppermint oil during stirring, and mix until semi-solidified.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com