Schiff base complex catalyst for synthesizing diphenyl carbonate through oxidative carbonylation of phenol
A technology of diphenyl carbonate and catalyst, applied in the field of preparation of diphenyl carbonate, can solve the problems of low DPC yield, complex catalyst preparation process and the like, and achieve the effects of short preparation time and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
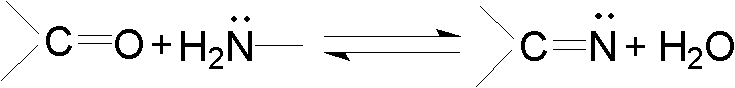
[0032] The general reaction formula of the preparation Schiff base ligand involved in the present invention is:
[0033]
Embodiment 1
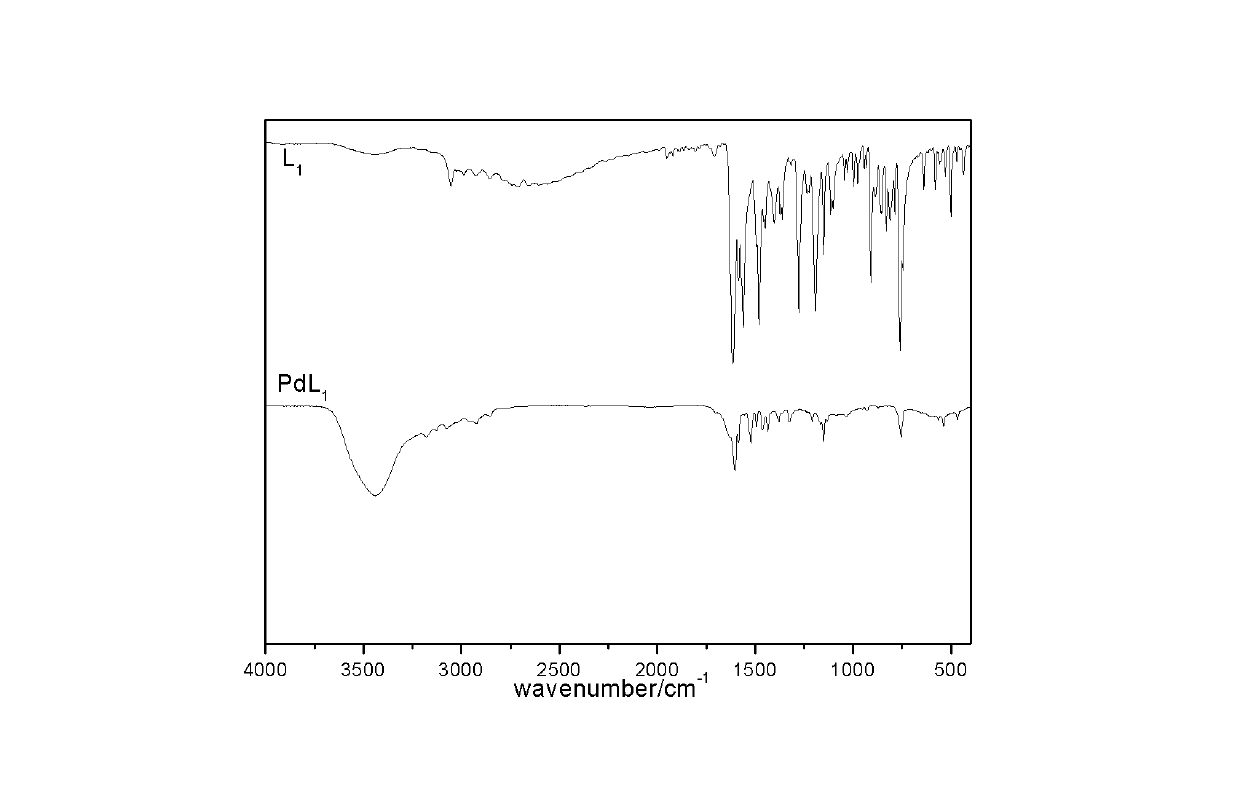
[0035] 1.0814g of o-phenylenediamine and 2.4424g of salicylaldehyde were respectively dissolved in 30mL of 90% absolute ethanol, and the ethanol solution of o-phenylenediamine was added dropwise to the ethanol solution of salicylaldehyde, and stirred at 60°C Reflux for 1 hour, and after cooling, columnar bright yellow crystals are precipitated, filtered, washed several times with absolute ethanol, and dried in a vacuum oven at 60°C to obtain o-phenylenediamine glycalaldehyde ligand L 1 .
[0036]Take 1mmol of ligand L 1 Dissolve in 30mL ethanol and tetrahydrofuran (volume ratio 2:1) mixed solvent, take 1mmol PdCl 2 Dissolve 2mmol KCl in 20mL double-distilled water, add the ligand solution drop by drop to the palladium solution, finish dripping for 10 minutes, stir thoroughly for 20 minutes, transfer to a microwave digestion tank, irradiate with microwaves at low fire for 3 minutes, and then follow the furnace Cool to room temperature, wash the precipitate with distilled wate...
Embodiment 2
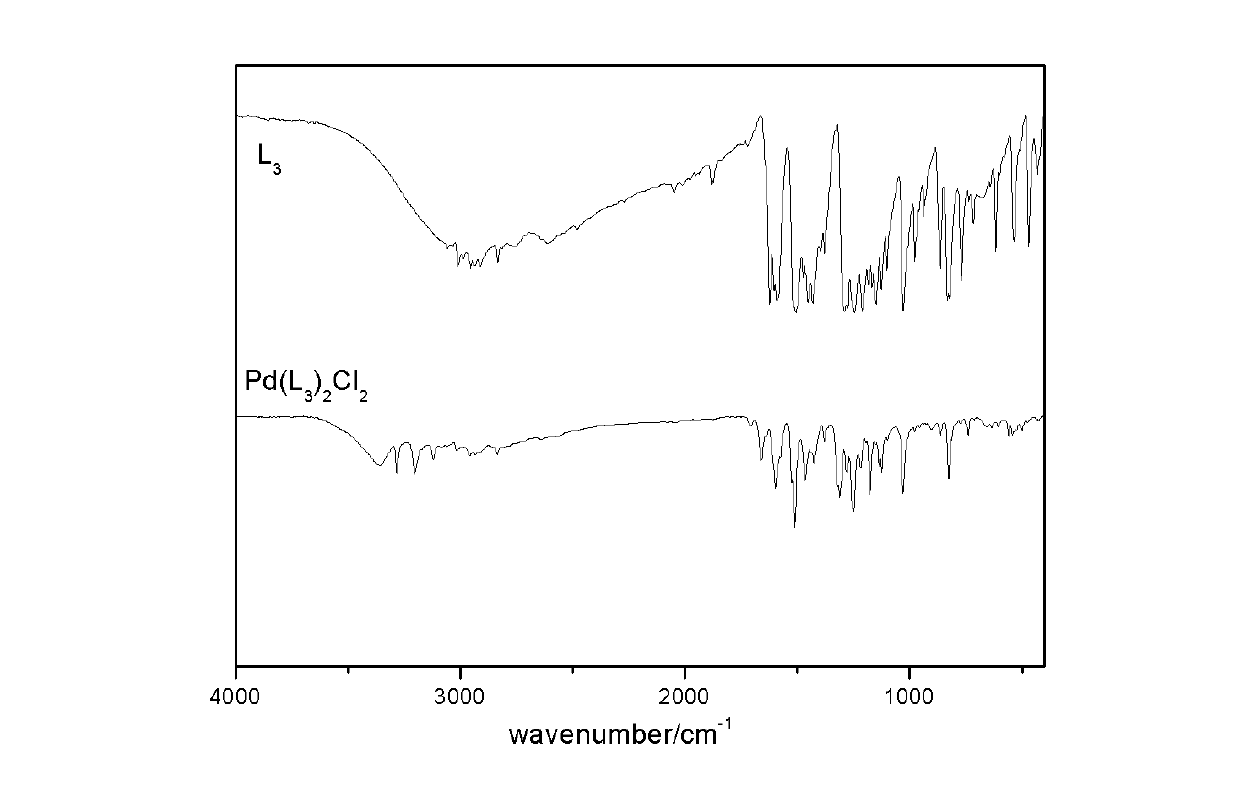
[0043] 1.2020g of ethylenediamine and 4.8848g of salicylaldehyde were respectively dissolved in 30mL of 95% absolute ethanol, and the ethanol solution of ethylenediamine was added dropwise to the ethanol solution of salicylaldehyde, and stirred and refluxed at 80°C for 1h , yellow crystals were precipitated after cooling, filtered, washed with absolute ethanol several times, and dried in a vacuum oven at 80°C to obtain the desired ligand ethylenediamine salicylaldehyde L 2 .
[0044] Take 1mmol of ligand L 2 Dissolve in 20mL ethanol and DMF (volume ratio 1:1) mixed solvent, take PdCl 2 : KCl=1:2 (molar ratio, PdCl 2 content of 1 mmol) was dissolved in 20 mL of double-distilled water, and the ligand solution was added dropwise to the palladium solution. After fully stirring for 20 min, it was transferred to a microwave digestion tank, microwaved at low heat for 3 min, and then cooled to room temperature with the furnace. , washed with distilled water and ethanol, and dried i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com