Method of adopting laminar duplex-metal hydroxide to recycle heavy metal ions in sewage
A layered bimetallic and heavy metal ion technology, applied in chemical instruments and methods, nickel oxide/nickel hydroxide, aluminum oxide/aluminum hydroxide, etc., to achieve good application prospects, fast adsorption rate, and uniform structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prepare 0.375mol / L Mg(NO 3 ) 2 ·6H 2 O and 0.125mol / L Al(NO 3 ) 3 9H 2 O mixed salt solution 100ml, prepare 100ml of sodium hydroxide and 0.3mol / L sodium nitrate mixed alkaline solution with deionized water. The mixed salt solution and the mixed alkali solution are dropped into the reaction vessel at the same time, and the pH is controlled to be 9.5-10. The reaction was vigorously stirred at room temperature for 8 h, during which time N 2 Protected, then centrifuged under the condition of 3500r / min, washed three times with deionized water, washed once with absolute ethanol, and dried to obtain layered double metal hydroxide. The chemical formula of the obtained layered double metal hydroxide is [Mg 0.75 2+ Al 0.25 3+ (OH) 2 ](NO 3 ) 0.25 1.5H 2 O.
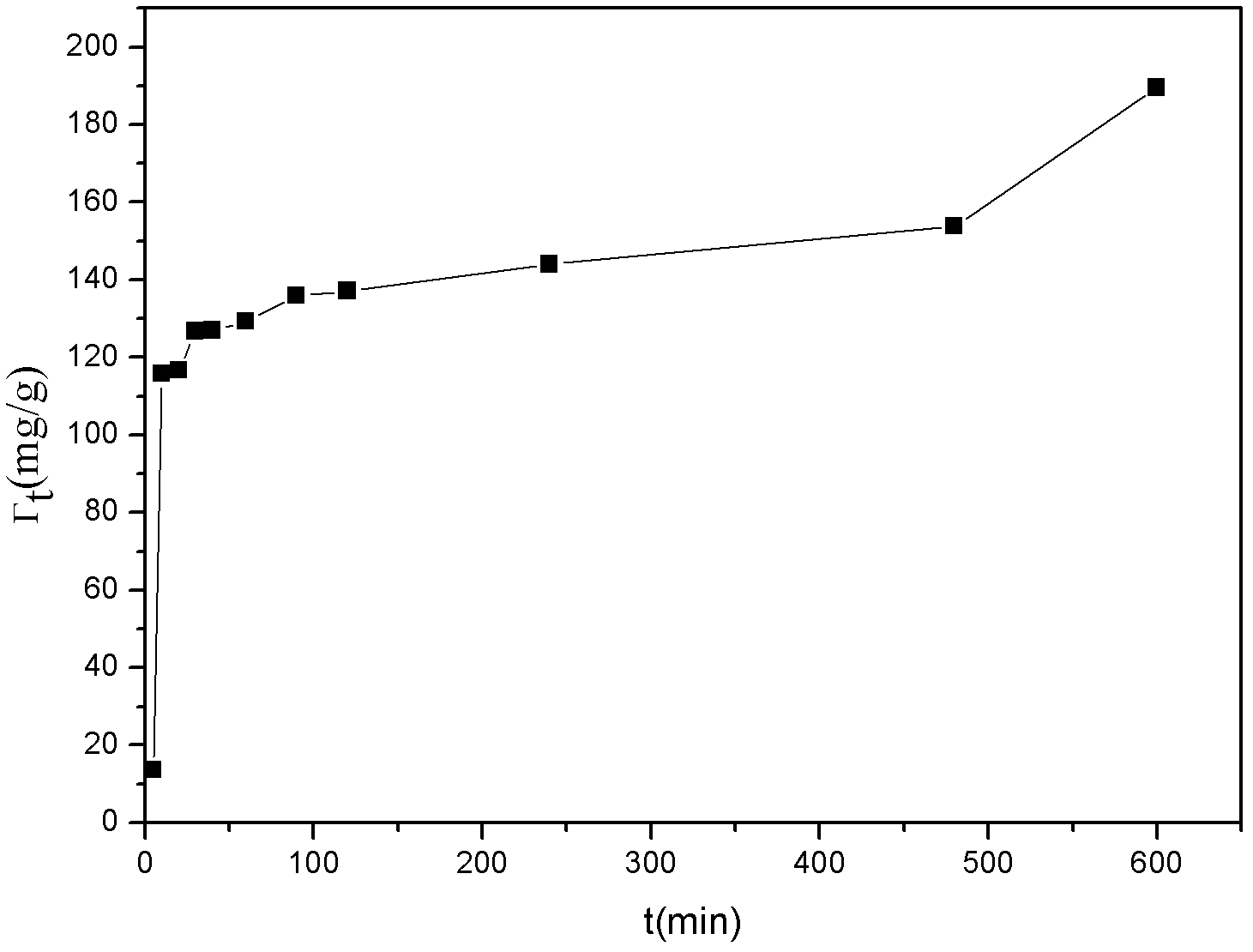
[0020] Cd 2+ Solution preparation: The country allows Cd that can discharge wastewater 2+ The concentration is 0.1mg / L, and the concentration of the solution to be tested is 10% of the national emission stan...
Embodiment 2
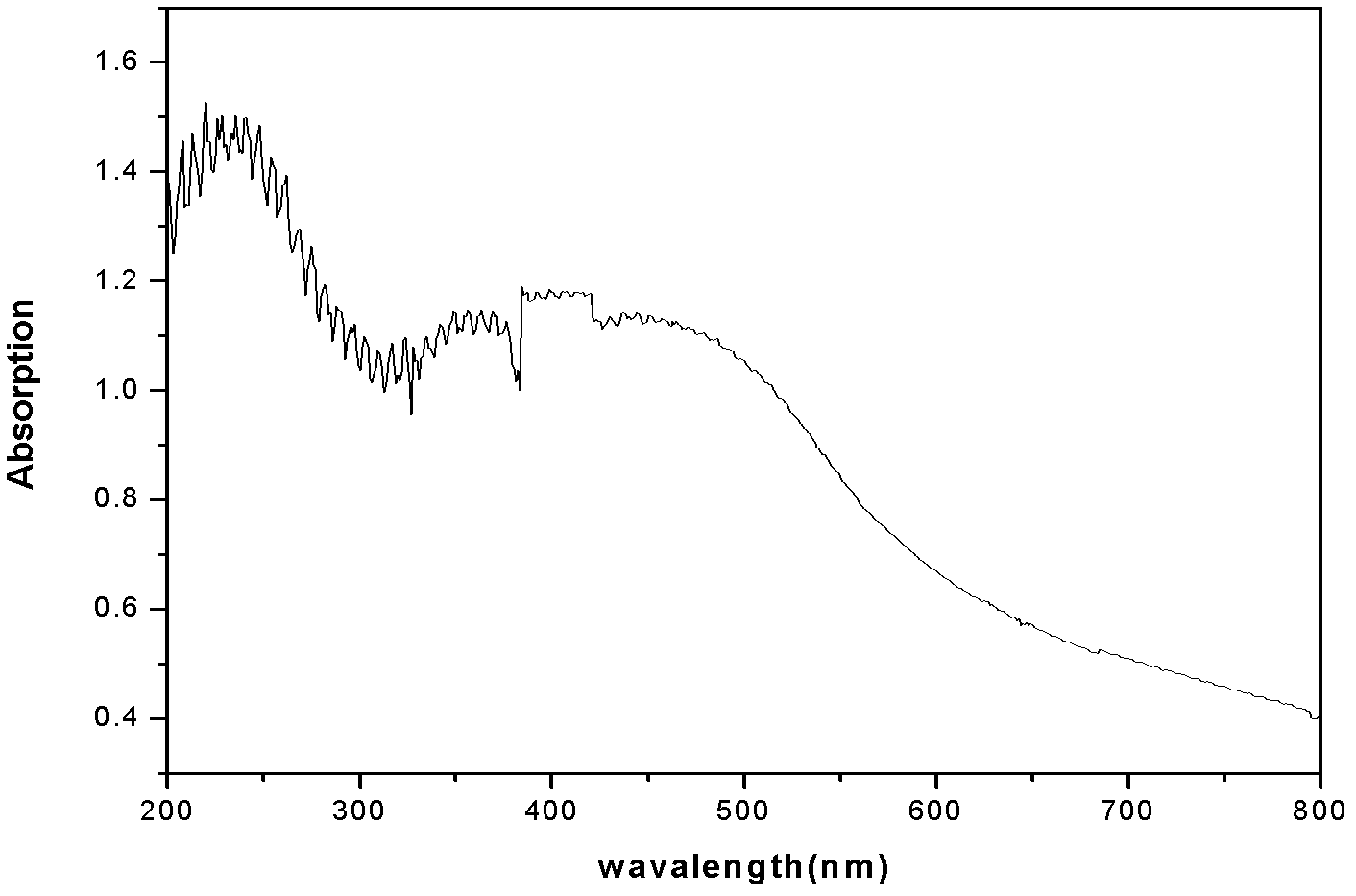
[0024] Using Ni(NO 3 ) 2 ·6H2 O replaces Mg(NO 3 ) 2 ·6H 2 O, all the other conditions are the same as in Example 1, and the preparation of chemical formula is [Ni 0.75 2+ Al 0.25 3+ (OH) 2 ](NO 3 ) 0.25 1.5H 2 layered double hydroxides of O, and Ni in the same way 2+ Adsorption, using ICP (plasma emission spectrometer) to measure Ni in the sample liquid to be tested 2+ The adsorption capacity was 58 mg / g. The adsorption product was calcined at 450°C to obtain a composite metal oxide. It also has obvious ultraviolet absorption performance in the range of λ<500nm.
Embodiment 3
[0026] Prepare 0.375mol / L Mg(NO 3 ) 2 ·6H 2 O and 0.125mol / L Al(NO 3 ) 3 9H 2 O mixed salt solution 100ml, prepare 100ml of sodium hydroxide and 0.3mol / L sodium carbonate mixed alkaline solution with deionized water. The mixed salt solution and the mixed alkali solution are dropped into the reaction vessel at the same time, and the pH is controlled to be 9.5-10. Vigorously stirred and reacted at room temperature for 8 hours, then centrifuged at 3500 r / min, washed three times with deionized water, once with absolute ethanol, and dried to obtain a layered double metal hydroxide. The chemical formula of the obtained layered double metal hydroxide is [Mg 0.75 2+ Al 0.25 3+ (OH) 2 ](CO 3 ) 0.125 2H 2 O.
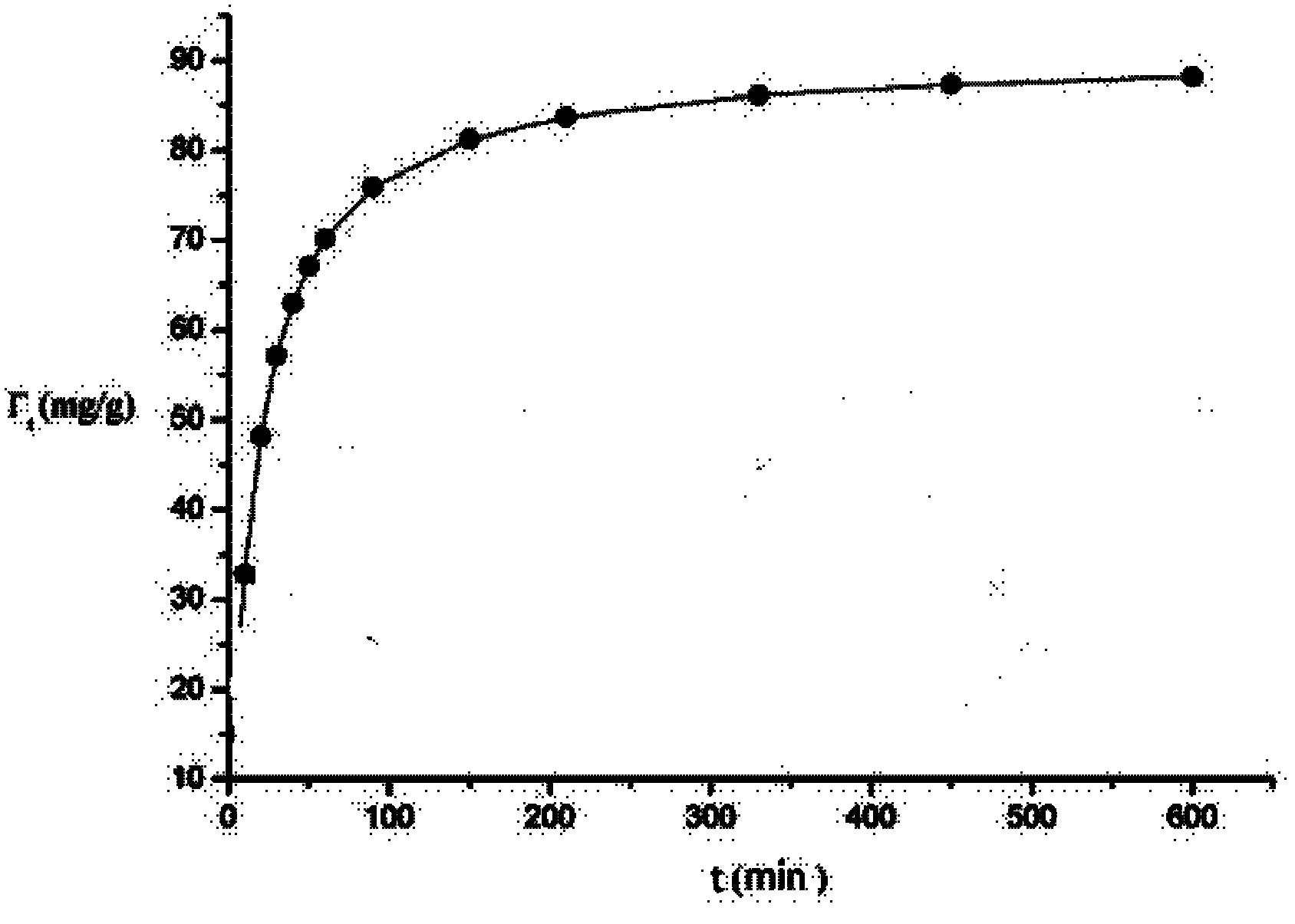
[0027] Ni 2+ Solution preparation: The country allows Ni that can discharge waste water 2+ The concentration is 0.5mg / L, and the concentration of the solution to be tested is 200 times that of the national discharge standard, that is, 0.5mg / L*200=0.1g / L, and 0.25g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com