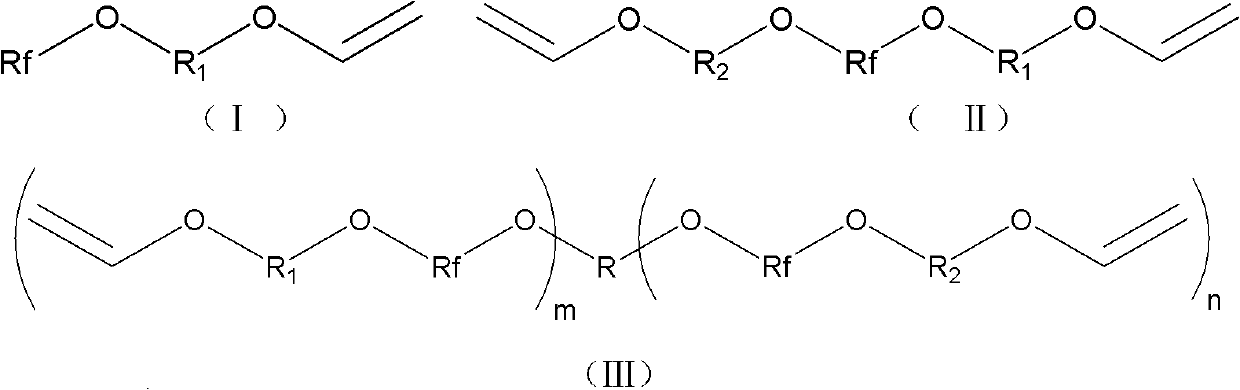
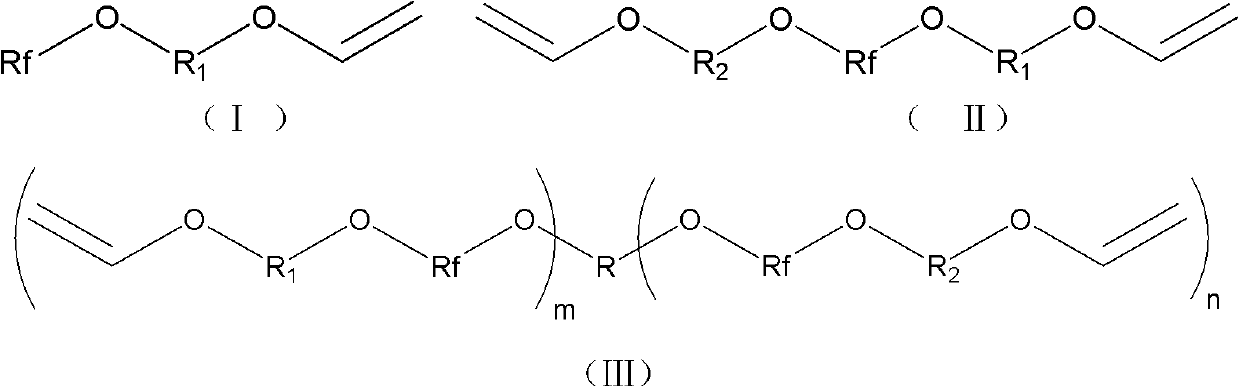
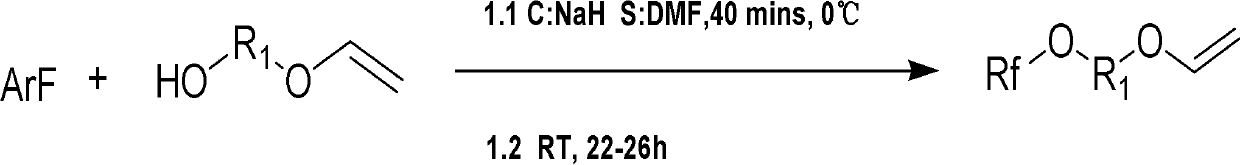
Fluorine contained aromatic polymerizing monomer with vinyl ether serving as end group and synthetic method
A technology of vinyl ether and synthetic method, which is applied in the direction of ether preparation, ester reaction to prepare ether, organic chemistry, etc. It can solve the problems of severe polymerization inhibition, poor surface curing, inconvenient operation, etc., and achieve the reduction of surface tension, storage and transportation Convenience, effects of improving weather resistance and chemical resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]Synthetic method of monomer A-1 (pentafluorophenoxyethyl vinyl ether):
[0067] Under anhydrous and oxygen-free conditions, 5.82g (66.1mmol) of ethylene glycol monovinyl ether and 2.64g (66.1mmol) of NaH were added to 50mL of DMF, placed in an ice-water bath, and mechanically stirred for 40min. A DMF (100 mL) solution of 12.29 g (66.1 mmol) of hexafluorobenzene was added dropwise, placed in an ice-water bath, and mechanically stirred for 40 min. The ice-water bath was removed, and stirring was continued at room temperature for 24 h. The reacted mixture was poured into ice water, extracted with diethyl ether (3*50mL), the obtained organic phase was washed with water (2*30mL), and then washed with anhydrous MgSO 4 dry. The diethyl ether was removed by rotary evaporation to obtain the crude product of the fluoromonomer as a pale yellow liquid. After the crude product was distilled under reduced pressure, it was left to stand for suction filtration, and then purified by c...
Embodiment 2
[0069] The synthesis method of monomer A-2 (pentafluorophenoxyethoxyethyl vinyl ether):
[0070] Use hydroxyethoxy ethyl vinyl ether to replace ethylene glycol monovinyl ether in [Example 1], and the remaining reagents and dosages are the same as [Example 1].
Embodiment 3
[0072] The synthesis method of monomer A-3 (pentafluorophenoxybutyl vinyl ether):
[0073] Use 4-hydroxybutyl vinyl ether to replace ethylene glycol monovinyl ether in [Example 1], and the remaining reagents and dosages are the same as [Example 1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com