Preparation method for ethoxyl sodium sulfonate fatty acid ester
A technology of sodium isethionate and fatty acid ester, applied in the preparation of sulfonate, organic chemistry and other directions, can solve the irritation of hydrogen chloride and phosphorus trichloride, and does not control the carbon number of sodium isethionate fatty acid ester Distribute color, odor, environmental pollution and other problems to achieve the effects of less environmental pollution, simple preparation method and rich source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
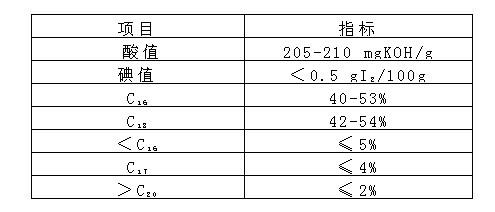
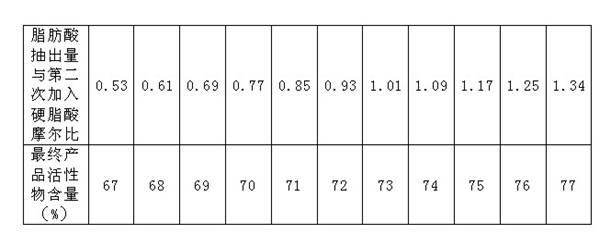
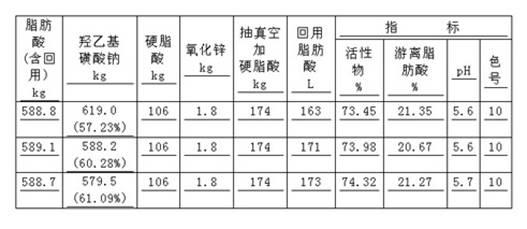
[0035]Raw materials are taken by proportioning amount, coconut oleic acid: 588.7kg melts ahead of time, sodium isethionate aqueous solution: 579.5kg (61.09%), vegetable type stearic acid: (addition amount is 106kg for the first time; Addition for the second time The amount is 174kg) melted in advance, zinc oxide ZnO: 1.8kg, 106kg of stearic acid and other raw materials are added to the reaction kettle, and the temperature is raised to 100-160°C for 90 minutes, and the temperature is raised to 221°C to start the esterification reaction. The reaction temperature is 240±2°C , the reaction time is maintained for 60 minutes, and then the reaction temperature is increased to 245°C for 10 minutes. The fatty acid evaporated during the esterification is condensed and refluxed into the reactor, and the vacuuming operation is started. When the vacuum degree reaches 30kPa, start to add the first The second part of stearic acid is 174kg, and the flow rate is controlled to add for 30-40 minu...
Embodiment 2
[0039] The raw materials were weighed, 513kg of coconut oleic acid was melted in advance, 540kg (57.23%) of sodium isethionate was dissolved in water, 89.7kg of plant-type stearic acid was melted in advance, and 1.42kg of zinc oxide ZnO was added to the reaction kettle and filled with nitrogen for protection. The temperature of the reaction kettle was raised to 100-160°C for 100 minutes, and the temperature was raised to 221°C to start the esterification reaction, and the temperature was continued to 240±2°C, and after 70 minutes of reaction, the reaction temperature was raised to 245°C and maintained for 10 minutes (the fatty acid evaporated during the esterification period was passed through condensed back into the reactor). Start to enter the vacuum operation, and blow nitrogen. When the vacuum reaches 30kPa, add 148kg of stearic acid for the second time, and the stearic acid addition time is 38 minutes. Take out 167L of fatty acid, make up nitrogen to make the reactor retu...
Embodiment 3
[0042] Take raw material by proportioning amount, coconut oleic acid 451.24kg melts ahead of time, sodium isethionate aqueous solution 464.79kg (58.62%), plant type stearic acid 79kg melts ahead of time, zinc oxide ZnO 1.25kg, add in the reactor, fill Nitrogen protection. The temperature of the reaction kettle was raised to 100-160°C for 90 minutes, and the temperature was raised to 221°C to start the esterification reaction, and the temperature was continued to rise to 240°C, maintained at 240±2°C for 60 minutes, and then the reaction temperature was raised to 245°C for a total reaction time of 90 minutes (during esterification The distilled fatty acid is refluxed into the reactor by condensation). Start vacuuming of the reactor, nitrogen bubbling, when the vacuum reaches 30kPa, add 130kg of stearic acid, add stearic acid for 30 minutes, the vacuum reaches 3kPa, the minimum temperature of the reactant drops to 234°C, take out 73L of fatty acid, replenish Nitrogen makes the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com