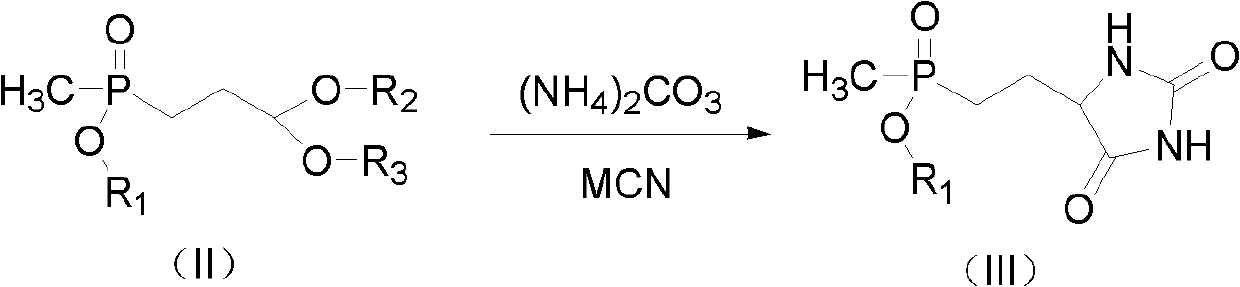
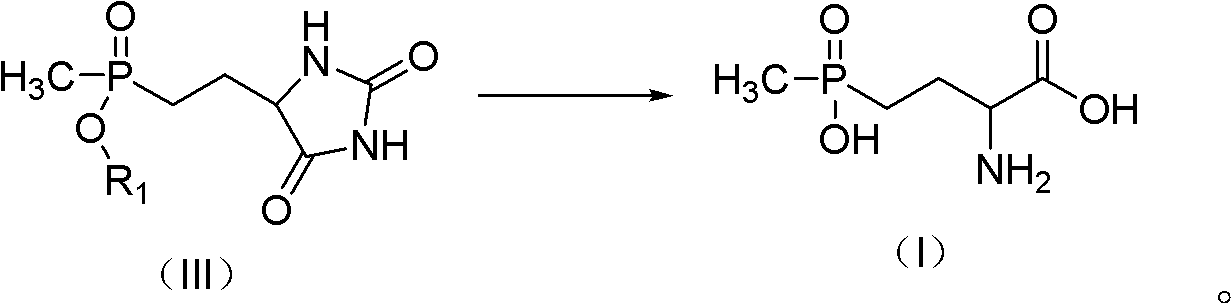
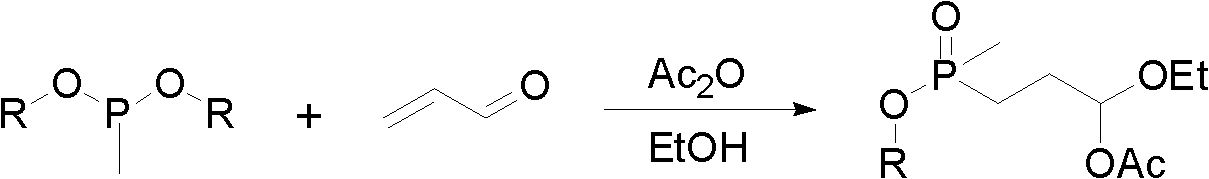
Preparation method for glufosinate
A technology of glufosinate-ammonium and ammonium carbonate is applied in the new preparation field of herbicide glufosinate-ammonium, which can solve the problems of harsh reaction conditions, expensive raw materials, low reaction yield and the like, and achieves the effects of mild conditions, easy detection and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of 5-(2-(methylethoxyphosphono) ethyl) hydantoin
[0028]
[0029] Add 25.2 g (0.1 mol) of ethyl (3-acetoxy-3-ethoxy) propylmethylphosphinate, 5.9 g (0.12 mol) of NaCN, and 100 ml of ethanol in succession into a 500 ml three-necked flask, and feed nitrogen After stirring at room temperature for 15 min, 100 ml of an aqueous solution containing 21.0 g (0.25 mol) of ammonium carbonate was added dropwise, and the temperature was raised to 70° C. for 3 h. The solvent and ammonium carbonate were removed by rotary evaporation at 80°C, and 100mL of ethanol was added to dissolve, filtered, and precipitated to obtain 24.5g of crude product 5-(2-(methylethoxyphosphono)ethyl)hydantoin. 87.2%, yield 91.3%. 1 H NMR (500MHz, DMSO): δ: 1.203-1.233 (m, 3H); δ: 1.409 (d, J=13.8Hz, 3H) δ: 1.653-1.888 (m, 4H); δ: 3.901-3.962 (m , 2H); δ: 4.081 (t, J=5.6Hz, 1H) δ: 7.988 (s, 1H); δ: 10.681 (s, 1H).
Embodiment 2
[0030] Embodiment 2: the preparation of 5-(2-(methylethoxyphosphono) ethyl) hydantoin
[0031] Add 25.2 g (0.1 mol) of ethyl (3-acetoxy-3-ethoxy) propylmethylphosphinate, 5.9 g (0.12 mol) of NaCN, and 100 ml of ethanol in succession into a 500 ml three-necked flask, and feed nitrogen After stirring at room temperature for 15 min, 100 ml of an aqueous solution containing 21.0 g (0.25 mol) of ammonium carbonate was added dropwise, and the temperature was raised to 70° C. for 1.5 h. The solvent and ammonium carbonate were removed by rotary evaporation at 80°C, and 100mL of ethanol was added to dissolve, filtered, and precipitated to obtain 23.5g of crude product 5-(2-(methylethoxyphosphono)ethyl)hydantoin, the content 79.1%, yield 79.4%.
Embodiment 3
[0032] Embodiment 3: the preparation of 5-(2-(methylethoxyphosphono) ethyl) hydantoin
[0033] Add 25.2 g (0.1 mol) of ethyl (3-acetoxy-3-ethoxy) propylmethylphosphinate, 5.9 g (0.12 mol) of NaCN, and 100 ml of ethanol in succession into a 500 ml three-necked flask, and feed nitrogen After stirring at room temperature for 15 min, 100 ml of an aqueous solution containing 21.0 g (0.25 mol) of ammonium carbonate was added dropwise, and the temperature was raised to 40° C. for 3 h. The solvent and ammonium carbonate were removed by rotary evaporation at 80°C, and 100mL of ethanol was added to dissolve, filtered, and precipitated to obtain 11.5g of crude product 5-(2-(methylethoxyphosphono)ethyl)hydantoin. 76.2%, yield 37.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com