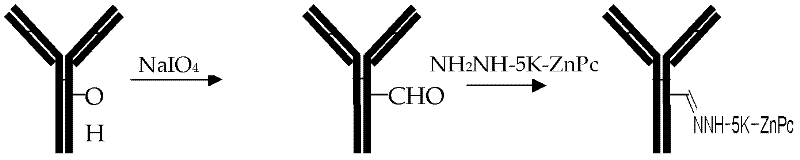
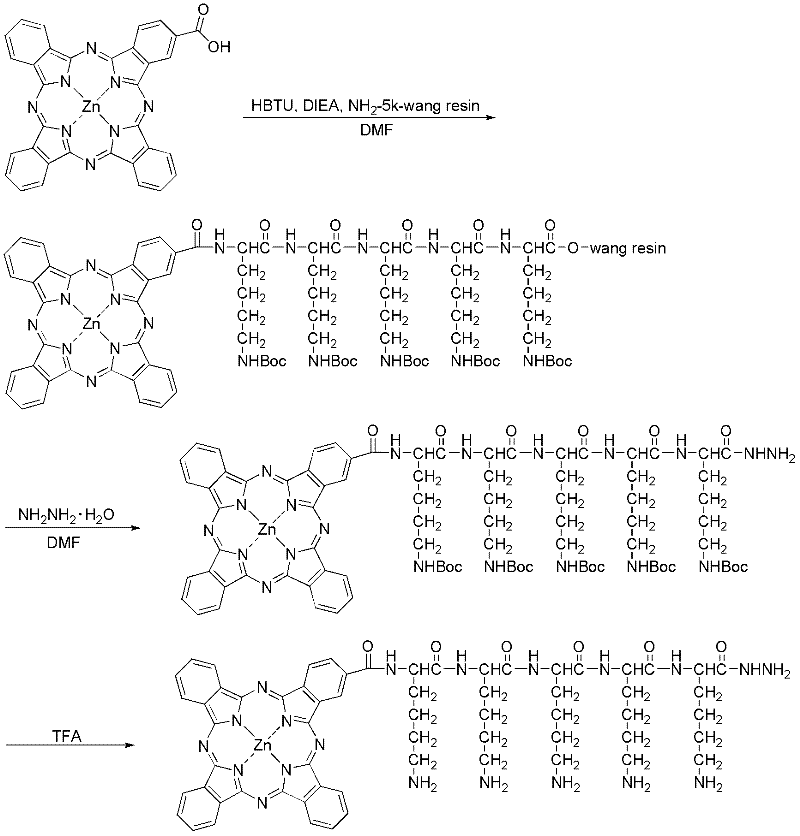
Preparation method and application of tumor-targeted photosensitive immunoconjugate
An immunoconjugate and tumor-targeting technology, which is applied to the preparation method of peptides, anti-tumor drugs, chemical instruments and methods, etc., can solve the problems of poor tumor selectivity, phototoxicity, and long-term light-shielding time for patients, and achieve High anti-tumor activity, good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 water-soluble single carboxyl phthalocyanine zinc hydrazide derivative ( figure 2 )
[0032] a. Weigh 20 mg monocarboxyzinc phthalocyanine (ZnPc-COOH), 24 mg benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate (HBTU), with pentameric lysine Resin 128mg of amino acid, pre-swelled in N,N-dimethylformamide (DMF) for 30min), added a total of 4ml of DMF (the reaction concentration of phthalocyanine is about 5mg / ml), and then dripped 5uL of N,N-di Isopropylethylamine (DIEA), stirred at room temperature, and reacted overnight in the dark, for a total of 16 hours. After the reaction is completed, filter with suction, wash with DMF until it is colorless, wash with methanol for 2-3 times, filter with suction and dry.
[0033] b. Take the above sample, add 5ml DMF, 200ul 85% hydrazine hydrate, stir at room temperature, avoid light overnight, then filter with suction, add a small amount of DMF to wash the resin, then add 10 times the...
Embodiment 2
[0036] The oxidation of embodiment 2 nimotuzumab
[0037] Take 40ul Nimotuzumab (5mg / ml, 0.033mmol / L), namely 200ug, add 10ul 50mmol / L sodium periodate (NaIO4) to make the final concentration reach 10mmol / L, room temperature (25°C water bath) After reacting in the dark for 30 minutes, add 10ul of 1mol / L ascorbic acid, and let it stand in a refrigerator at 4°C for 30 minutes to remove residual NaIO4 for later use.
Embodiment 3
[0038] Nimotuzumab after embodiment 3 oxidation is coupled with ZnPC-CO-5K-NHNH2
[0039] ZnPC-CO-5K-NHNH2 is prepared into a 3.3mmol / L stock solution with pure water, take the oxidized Nimotuzumab, add 10ul of the above stock solution per 100ul, add 10ul 1mol / L phosphate buffered saline ( PH7.2), mix well, and vortex shake in the dark at 4°C refrigerator overnight, add 20ul 1mol / L sodium borohydride (NaBH4) (1mol / L Na2CO3 freshly prepared) solution, 4°C refrigerator, stand still for 4h. Separate with SephadexG100 gel column (12×300mm), use PBS as eluent, collect the first elution peak, and concentrate to 100ul with a 10kDa ultrafiltration centrifuge tube for use. At the same time, the purity was detected by 10% polyacrylamide gel electrophoresis with non-reducing SDS-page ( image 3 , Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com