Poly-((methyl) crylic acid-b-styrene-b-butadiene-b-styrene) segmented copolymer latex and preparation method thereof
A technology of block copolymer and methacrylic acid, which is applied in the field of polymers, can solve the problems of low final conversion rate, slow reaction speed, long reaction polymerization inhibition period, etc., and achieve the effects of low viscosity, small pollution and fast heat transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
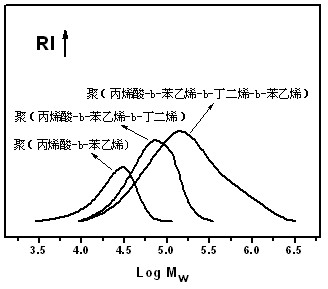
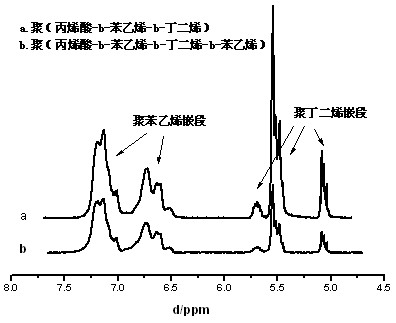
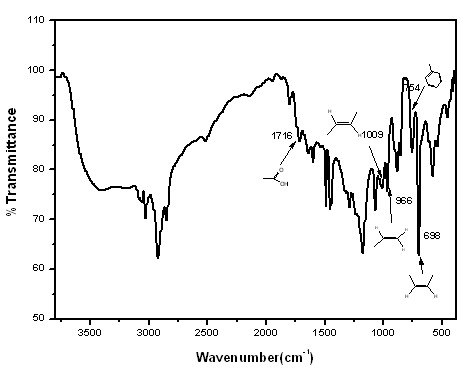
[0034] Add 80 parts by weight of water, 2 parts by weight of amphiphilic macromolecular reversible addition-fragmentation chain transfer reagent macroRAFT1 (its chemical structure is shown below by proton nuclear magnetic resonance analysis), and 10 parts by weight of styrene into the reactor, Stir and mix, blow nitrogen for 30 minutes, heat up to 70°C, add 0.04 parts by weight of potassium persulfate, react for 45 minutes, add 0.5 parts by weight of sodium hydroxide, add 0.5 parts by weight of sodium dodecyl sulfate (SDS) , after fully mixing, add 30 parts by weight of butadiene, add 0.005 parts by weight of potassium persulfate, react for 50 minutes, slowly release the pressure, discharge unreacted butadiene from the reactor, and then add 10 parts by weight of benzene Ethylene, continue to react for 30 minutes, cool and discharge, add terminator hydroquinone THF solution (1%) to obtain block copolymer latex. figure 1 Showing the evolution of the GPC molecular weight of the r...
Embodiment 2
[0038] Add 100 parts by weight of water, 1 part by weight of amphiphilic macromolecular reversible addition-fragmentation chain transfer reagent macroRAFT2 (its chemical structure is shown below by proton nuclear magnetic resonance analysis), and 10 parts by weight of styrene into the reactor, Stir and mix, blow nitrogen for 60 minutes, heat up to 70°C, add 0.04 parts by weight of potassium persulfate, react for 45 minutes, add 0.5 parts by weight of sodium hydroxide, add 0.4 parts by weight of sodium dodecyl sulfate (SDS ), after fully mixing, add 20 parts by weight of butadiene, add 0.02 parts by weight of potassium persulfate, react for 4 hours, slowly release the pressure, discharge unreacted butadiene from the reactor, and then add 10 parts by weight of Styrene, continue to react for 30 minutes, cool and discharge, add terminator hydroquinone THF solution (1%) to obtain block copolymer latex. The design and measured molecular weight of each block of the obtained block cop...
Embodiment 3
[0042] 40 parts by weight of water, 0.2 parts by weight of amphiphilic macromolecular reversible addition-fragmentation chain transfer reagent macroRAFT1 (its chemical structure is the same as shown in Example 1), and 4 parts by weight of styrene are added to the reactor, stirred and mixed, and passed Nitrogen for 30 minutes, heat up to 70°C, add 0.04 parts by weight of potassium persulfate, react for 45 minutes, add 0.5 parts by weight of sodium hydroxide, add 0.1 parts by weight of sodium dodecyl sulfate (SDS), and mix well Finally, add 18 parts by weight of butadiene, add 0.1 parts by weight of potassium persulfate, react for 6 hours, slowly release the pressure, discharge unreacted butadiene from the reactor, add 4 parts by weight of styrene, and continue the reaction After 30 minutes, the material was cooled and discharged, and a terminator hydroquinone THF solution (1%) was added to obtain the latex of the block copolymer. The design and measured molecular weight of each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com