Novel process for co-production of liquefied synthesis gas, pure hydrogen and methanol from coke-oven gas
A technology for synthesizing natural gas and coke oven gas, which is applied in gas fuel, organic chemistry, petroleum industry, etc. It can solve the problems of overcapacity, loss of coke oven gas, large investment, etc., and achieve the effect of avoiding high temperature and carbon formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
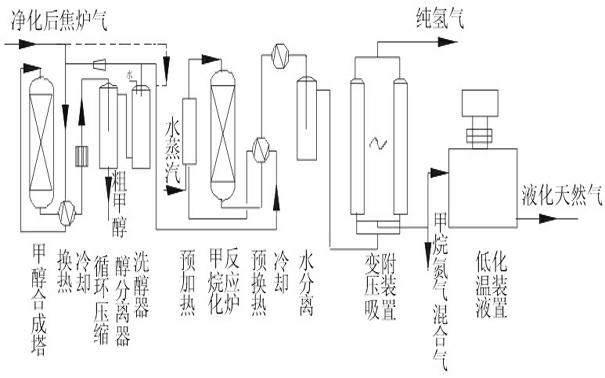
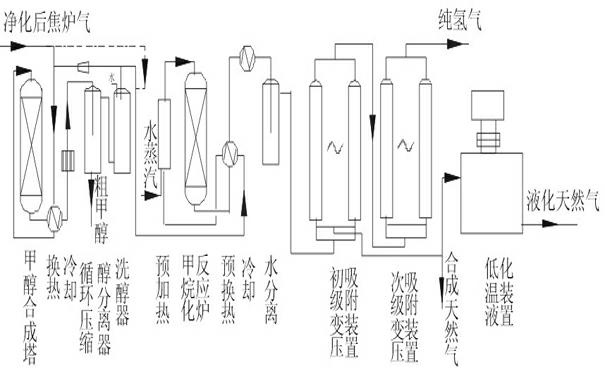
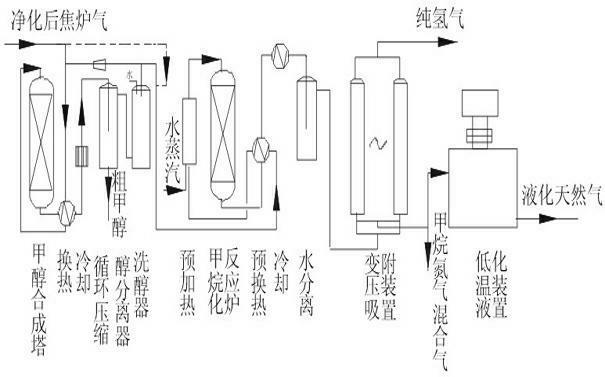
[0038] Such as figure 1 As shown, the purified coke oven gas is compressed and pressurized to 5MPa, and the main component content of coke oven gas is calculated as: H 2 56%, CH 4 27%, N28.6%, CO 6.2%, CO 2 2.2%, and other small components can be ignored. The crude methanol product is obtained through heat exchange and into the methanol synthesis tower, and the crude methanol is refined through rectification to obtain refined methanol. The methanol synthesis catalyst adopts XNC-98 catalyst, and the CO content is reduced by circulation. to 1.5%, CO 2 After reducing to 2%, it is sent to the methanation heat exchange, preheating and methanation adiabatic catalytic reactor. The inlet of the methanation reactor is 250°C, and the outlet is about 440°C. It can react the total amount of COx to about 8PPM, including 2 Carbon atoms (carbon two) and hydrocarbons with more than 2 carbon atoms will be hydrogenated into methane, and after heat exchange, cooling and water separation, H...
Embodiment 2
[0040] Such as figure 1 As shown, the purified coke oven gas is compressed and pressurized to 2MPa, and the main component content of coke oven gas is calculated as: H 2 56%, CH 4 27%, N28.6%, CO 6.2%, CO 2 2.2%, and other small components can be ignored. The crude methanol product is obtained through heat exchange and into the methanol synthesis tower, and the crude methanol is refined through rectification to obtain refined methanol. The methanol synthesis catalyst adopts XNC-98 catalyst, and the CO content is reduced by circulation. to 1.5%, the CO2 is reduced to 2%, and sent to the methanation heat exchange, preheating and methanation adiabatic catalytic reactor. The inlet of the methanation reactor is 250 ° C, and the outlet is about 440 ° C. 10PPM, C2 and above hydrocarbons will be converted to methane by hydrogenation, after heat exchange, cooling and water separation, the H2 containing 47%, CH 4 41%, Yu N 2 The mixed gas, the pure hydrogen product (more than 99...
Embodiment 3
[0042] Such as figure 1 As shown, the purified coke oven gas is compressed and pressurized to 10MPa, and the main component content of coke oven gas is calculated as: H 2 56%, CH 4 27%, N28.6%, CO 6.2%, CO 2 2.2%, and other small components can be ignored. The crude methanol product is obtained through heat exchange and into the methanol synthesis tower, and the crude methanol is refined through rectification to obtain refined methanol. The methanol synthesis catalyst adopts XNC-98 catalyst, and the CO content is reduced by circulation. After CO2 drops to 1.8%, it is sent to the methanation heat exchange, preheating and methanation adiabatic catalytic reactor. The inlet of the methanation reactor is 250°C, and the outlet is about 430°C. The total amount of COx can be reacted to 5PPM, hydrocarbons containing 2 carbon atoms or more will be hydrogenated into methane, after heat exchange, cooling and water separation, a mixed gas containing 47% of H2, 41% of CH4 and remaining...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com