Infusion preparation for dialysis patient
A technology for patients and uses, applied in blood diseases, drug delivery, extracellular fluid diseases, etc., to achieve the effect of reducing the dosage and frequency of occurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1: Preparation of anti-anemia preparation (amino acid infusion)
[0091] The ingredients shown in the following Table 1 were dissolved in water for injection according to the amounts shown in the table. The solution was sterile filtered and placed in a glass bottle. The bottle was sealed and autoclaved to prepare an anti-anemia preparation (amino acid infusion).
[0092] The pH value of the preparation is 6.6-7.6, and the osmotic pressure ratio is about 2.
[0093] Table 1:
[0094] Element
[0095] The amino acid infusion prepared according to Example 1 is also used as the following purposes: a pharmaceutical preparation for reducing the dosage of erythropoietin (second aspect of the present invention), a pharmaceutical preparation for controlling serum phosphorus levels (third aspect of the present invention) and Pharmaceutical preparations for inhibiting protein catabolism (fourth aspect of the invention).
Embodiment 2
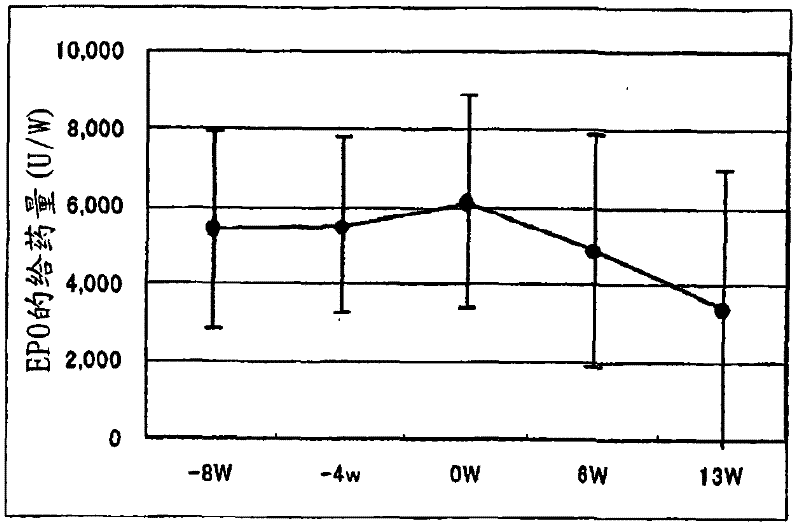
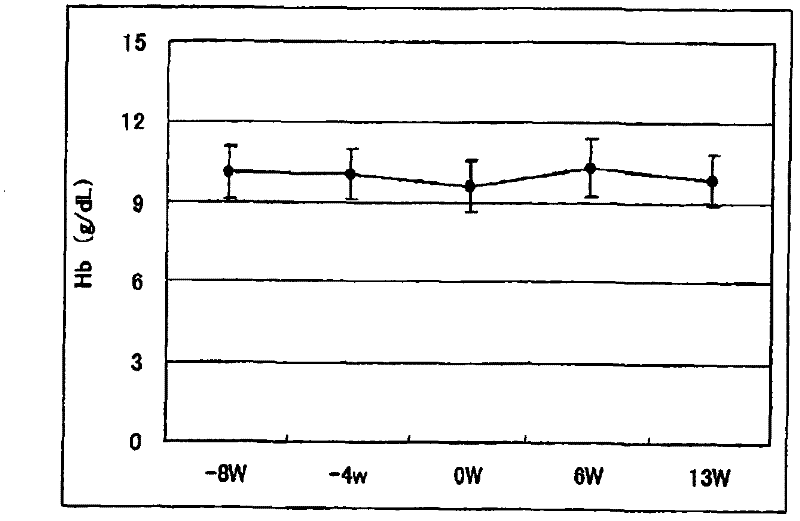
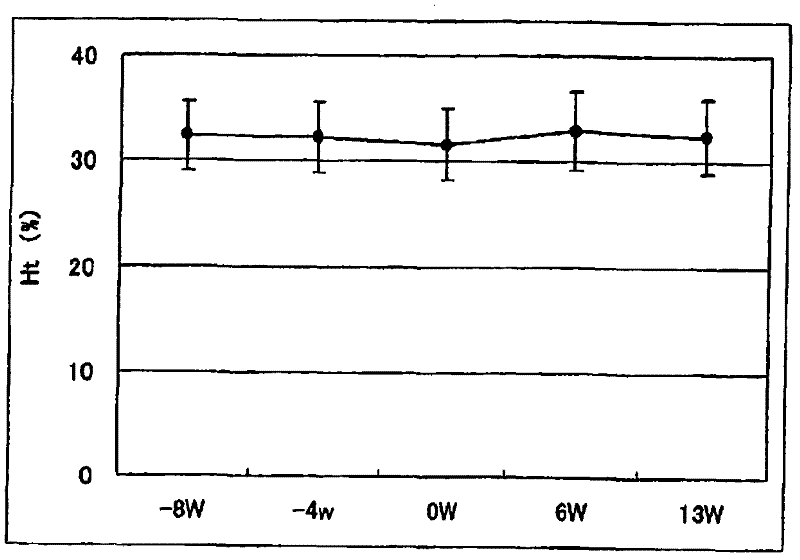
[0096] Example 2. Determination of the ability of pharmaceutical preparations to improve anemia and reduce the dosage of erythropoietin
[0097] Exemplary anti-anemia preparation of the present invention was administered to 18 chronic renal failure patients receiving hemodialysis (HD: 9 cases) or hemodiafiltration (HFD: 9 cases) to examine that the preparation improves anemia and reduces erythropoietin administration Quantitative capacity.
[0098] [method]
[0099] A commercially available amino acid infusion solution "NEOAMYU (registered trademark)" (200 mL formulation, Ajinomoto Pharma Co., Ltd.) prepared according to Example 1 was used.
[0100] In the week before the administration period, the necessary diagnosis, examination and detection were carried out on the first dialysis day (week 1 or week 2). The "NEOAMYU" preparation was continuously administered from 1 week after the diagnosis to 12 weeks and 1 day (the 37th dialysis). Specifically, 1 vial (200 mL) of the pr...
Embodiment 3
[0107] Example 3: Determination of the ability of the above pharmaceutical formulations to control serum phosphorus levels
[0108] The exemplary serum phosphorus control preparation of the present invention was administered to 8 chronic renal failure patients receiving hemodialysis (HD) to examine changes in serum phosphorus levels.
[0109] [method]
[0110] The commercially available amino acid infusion solution "NEOAMYU" (200 mL formulation, Ajinomoto Pharma Co., Ltd.) prepared according to Example 1 was used.
[0111] In the week before the administration period, the necessary diagnosis, examination and detection were carried out on the first dialysis day (week 1 or week 2). The "NEOAMYU" preparation was continuously administered from 1 week after the diagnosis to 12 weeks and 1 day (the 37th dialysis). Specifically, 1 vial (200 mL) of the preparation was infused into the venous side of the dialysis circuit each time of dialysis. The formulation was infused throughout ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com