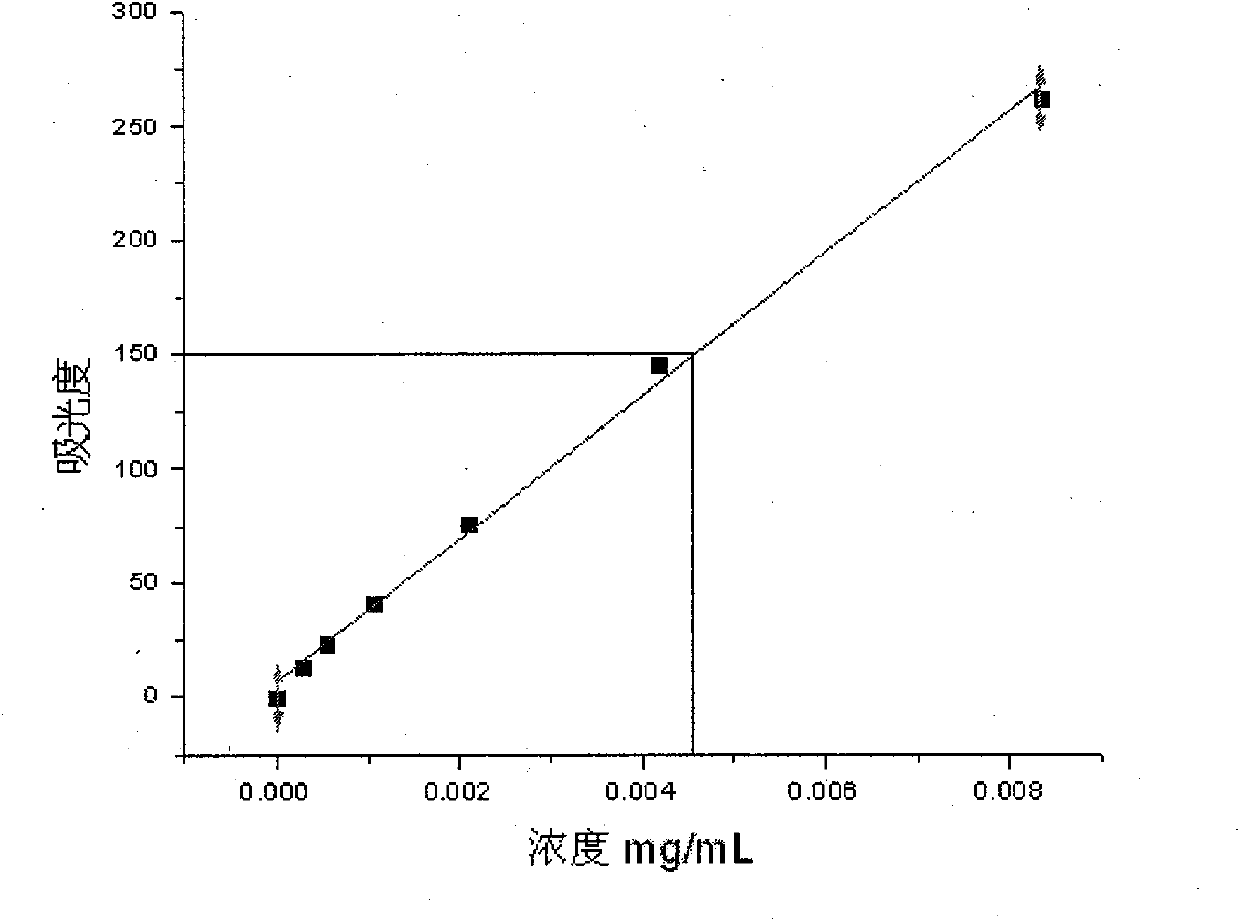
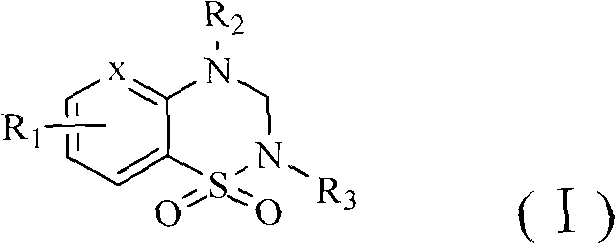
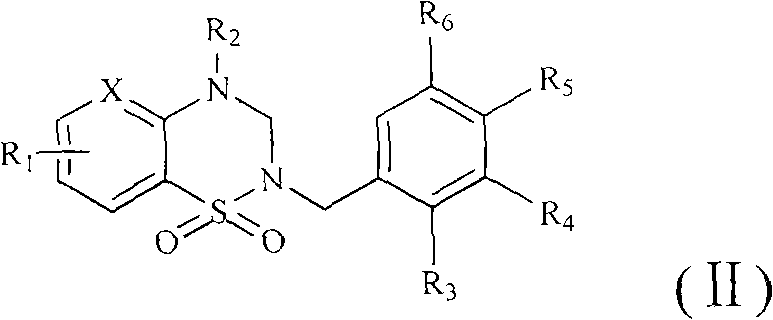
Structure utilizing benzothiadiazine and pyrazolothiadiazine derivatives as aldose reductase inhibitor, synthetic method and application
A technology of compound structure and use, applied in drug combination, metabolic diseases, organic chemistry, etc., can solve problems such as poor therapeutic effect, low bioavailability, side effects and allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] Add 2-aminobenzenesulfonamide (17.2g, 100mmol) and 150mL triethyl orthoformate 150mL into the round bottom flask, stir and reflux for 2h, after returning to room temperature, filter, the filter cake is washed with anhydrous ether, and dried to obtain 4H- 1,2,4-benzothiadiazine 1,1-dioxo (16.2g, 89%): melting point: 226-228°C; 1 HNMR (400MHz, DMSO-d 6 ): δ7.28(m, 1H), 7.43(m, 1H), 7.65(m, 1H), 7.78(m, 1H), 7.96(s, 1H), 12.28(s, 1H).
[0082] A round bottom flask was charged with 4H-1,2,4-benzothiadiazine 1,1-dioxo (14.6 g, 80 mmol), potassium carbonate (12 g), methyl bromoacetate (13.4 g, 88 mmol) and acetonitrile (180 mL), react at 70°C for 2 h, remove the solvent by rotary evaporation, filter, wash repeatedly with water, and dry. The crude product was recrystallized from ethyl acetate to give white crystals of methyl (4H-1,2,4-benzothiadiazine-1,1-dioxo-4-alkyl)acetate (16.9 g, 83%): mp : 154-156°C; 1 H NMR (400MHz, DMSO-d 6 ): δ3.74(s, 3H), 5.10(s, 2H...
Embodiment 2
[0087]
[0088] Substitute 3-nitrobenzyl bromide for 4-bromo-2-fluorobenzyl bromide in Example 1, and prepare according to the preparation method described in Example 1.
[0089] Melting point: 166-169°C; 1 H NMR (400MHz, DMSO-d 6 ): δ4.20(s, 2H), 4.42(s, 2H), 4.95(s, 2H), 6.76(d, 1H), 6.89(m, 1H), 7.46(m, 1H), 7.65(m, 2H), 8.17(m, 1H), 8.20(m, 2H), 12.98(s, 1H); elemental analysis (C 16 h 15 N 3 o 6 S): theoretical value C: 50.92%, H: 4.01%, N: 11.13%; actual value C: 51.00%, H: 4.05%, N: 11.12%.
[0090] Example 3 [2-(4-trifluoromethylbenzyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxo-4-alkyl]acetic acid (compound 3 )
Embodiment 3
[0091]
[0092] Substitute 4-trifluoromethylbenzyl bromide for 4-bromo-2-fluorobenzyl bromide in Example 1, and prepare according to the preparation method described in Example 1.
[0093] Melting point: 152-155°C; 1 H NMR (400MHz, CDCl 3 ): δ3.98(s, 2H), 4.39(s, 2H), 4.79(s, 2H), 6.58(d, 1H), 6.96(m, 1H), 7.43(m, 1H), 7.51(d, 2H), 7.62(d, 2H), 7.78(m, 1H); elemental analysis (C 17 h 15 f 3 N 2 o 4 S): theoretical value C: 51.00%, H: 3.78%, N: 7.00%; actual value C: 50.64%, H: 3.88%, N: 6.93%.
[0094] Example 4 [2-(2,4,5-trifluorobenzyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxo-4-alkyl]acetic acid ( Compound 4)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com