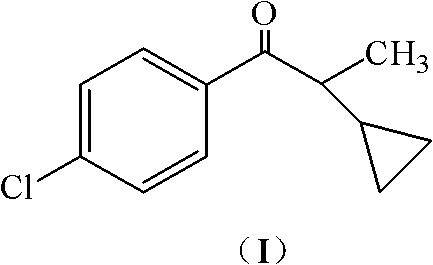
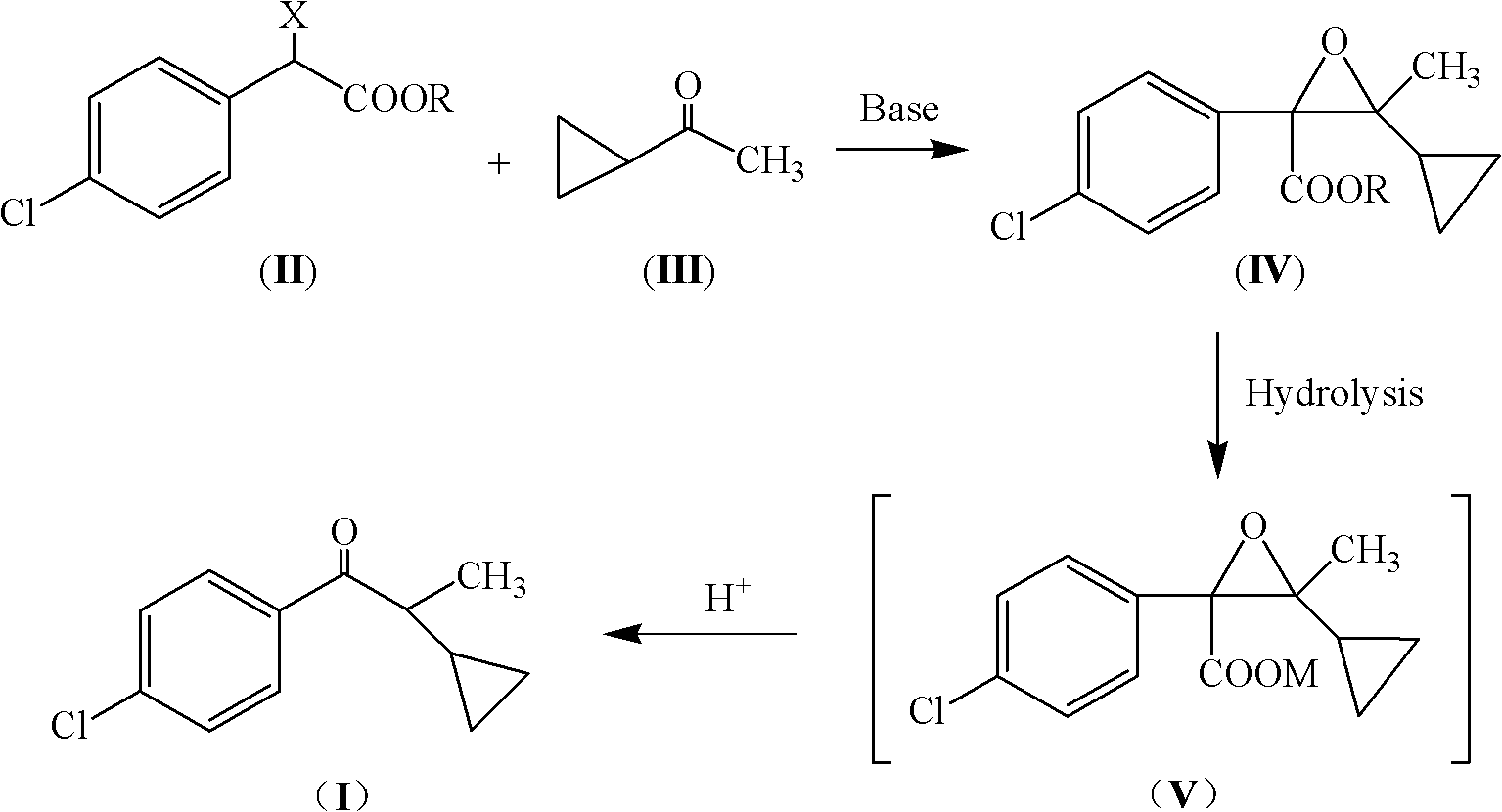
Preparation method of 1-(4-chlorophenyl)-2-cyclopropyl-1-acetone and intermediate thereof as well as preparation method of intermediate
A technology of cyclopropyl methyl ketone and cyclopropyl, which is applied in the preparation of carbon-based compounds, heterocyclic compounds, organic compounds, etc., can solve the problems of major safety problems, large amount of boron trifluoride, etc., Achieve the effect of high product content and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
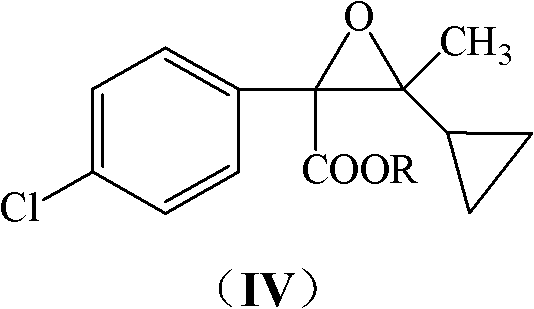
[0041] The sodium tert-butoxide solution prepared from 5.6g sodium metal and 150mL tert-butanol was added dropwise to 17.0g cyclopropyl methyl ketone and 48.0g α-chloro-p-chlorophenylacetic acid methyl ester under ice-water bath cooling In the mixture, control the temperature of the system between 15°C and 20°C; after the addition, stir and react at 20°C to 30°C for 4 hours; evaporate most of the tert-butanol under reduced pressure, and after cooling to room temperature, add 100mL of water and 100mL of di Chloromethane, static until the layers were separated, the aqueous layer was extracted with 30 mL×2 dichloromethane, the combined organic layers were washed with water and dried over anhydrous magnesium sulfate to obtain methyl glycidate (IV, R=methyl) solution.
[0042] After desolventizing the above solution, methyl glycidate IV (R=methyl) can be obtained: EIMS (m / z): 268 (M+2), 266 (M); 1 H NMR: δ0.20-0.50(m, 4H), 0.71(m, 1H), 1.34(s, 3H), 3.69(s, 3H), 7.13(d, J=8.1Hz, 2H)...
Embodiment 2
[0046] Use 8.0g potassium tert-butoxide, 35mL tert-butanol, 4.2g cyclopropyl methyl ketone and 13.0g α-chloroethyl p-chlorophenylacetate as raw materials, refer to the method of Example 1, obtain 12.2g Ethyl glycidate (IV, R=ethyl): EIMS (m / z): 282 (M+2), 280 (M); 1 H NMR: δ0.20-0.50(m, 4H), 0.72(m, 1H), 1.30(t, J=6.3Hz, 3H), 1.33(s, 3H), 4.09(q, J=6.3Hz, 2H ), 7.15 (d, J=8.2Hz, 2H), 7.22 (d, J=8.2Hz, 2H).
Embodiment 3
[0048] The glycidic acid methyl ester (IV, R=methyl) solution obtained by repeating Example 1 was added dropwise to a solution composed of 18.0g potassium hydroxide and 120mL methanol, and the resulting mixture was stirred at room temperature for 6 hours; under cooling in an ice-water bath, Slowly add 120mL of water and stir for 2 hours; then add dropwise 30% hydrochloric acid to adjust the pH to 3~4, and continue to stir for 4 hours at room temperature; let the layers stand, extract the aqueous layer with 40mL of dichloromethane, combine the organic layers, and add 5% bicarbonate Wash with aqueous sodium solution, then wash with water until neutral, and remove the solvent to obtain 34.2 g of crude product 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (I), which is a translucent red liquid with a chromatographic analysis content of 93.4% , yield 76.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com