S-type and R-type tetrahydro-naphthalene amides antitumor compound and pharmaceutically acceptable salt or pro-drug thereof, preparation method and application
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as life-threatening and serious conditions of patients, and achieve the effect of wide therapeutic window, broad anti-cancer spectrum, excellent anti-tumor activity and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
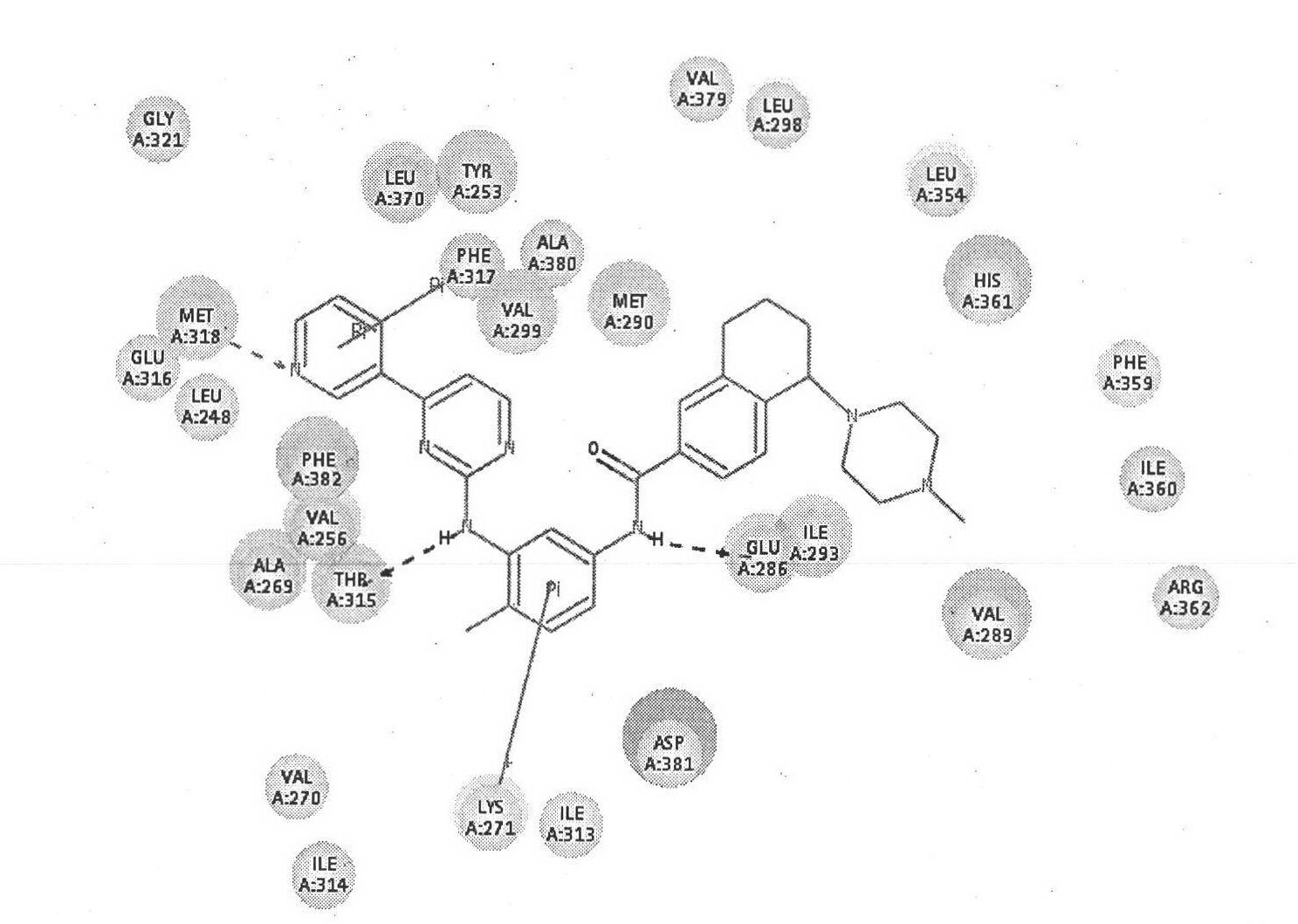
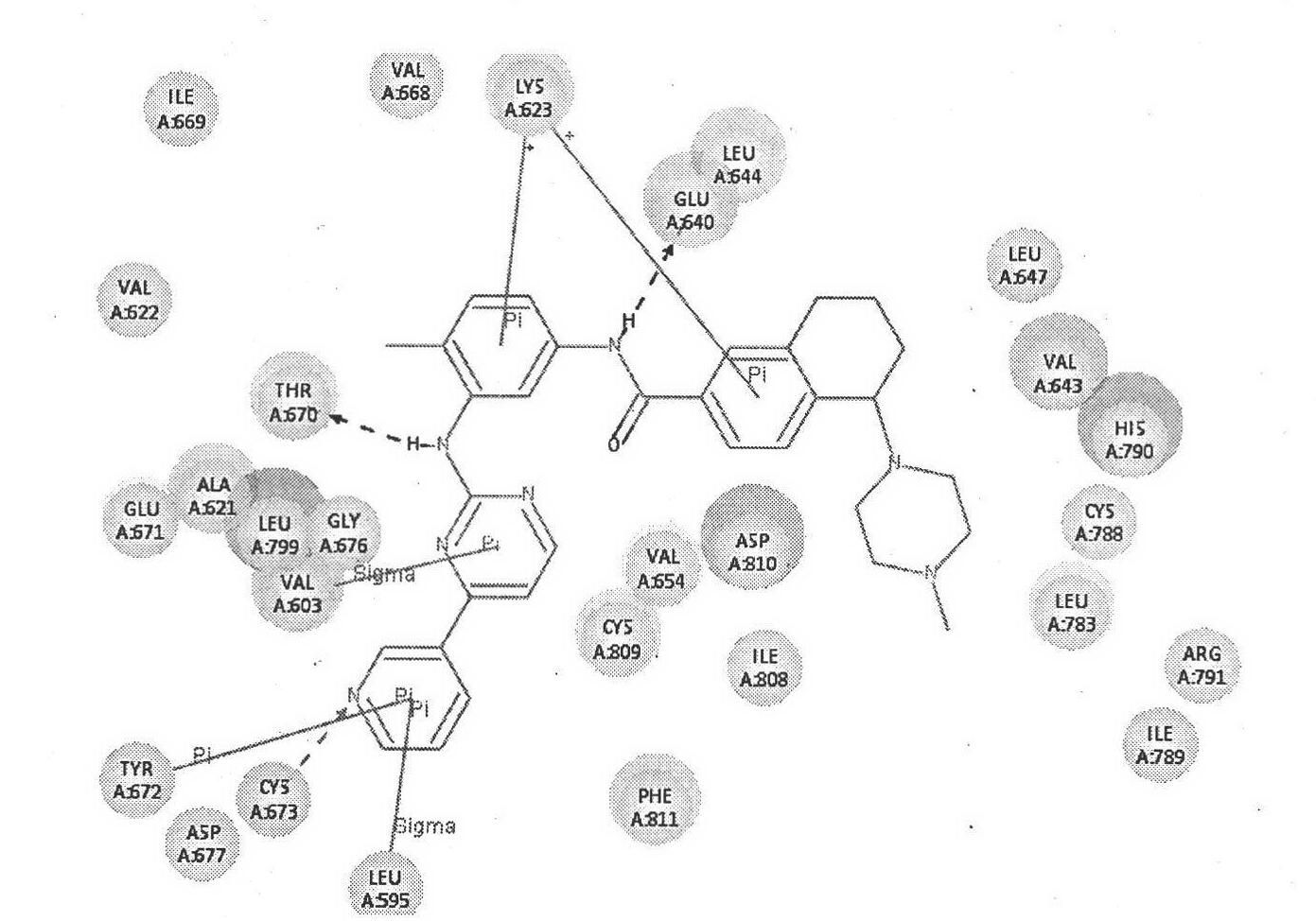
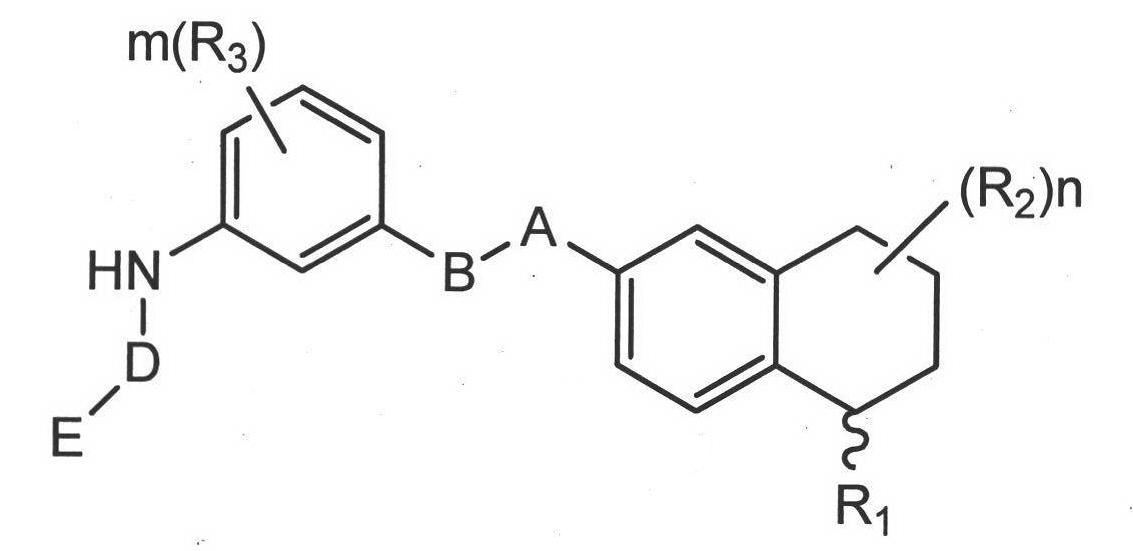
Image
Examples
Embodiment 1
[0090] Example 1: (S)-N-{4-methyl-3-[(4-pyridin-3 base)pyrimidine-2-amino]phenyl}-5-(4-methylpiperazin-1-yl )-5,6,7,8-tetrahydronaphthalene-2-amide preparation
[0091]
[0092] Step A: Synthesis of (S)-methyl 5-hydroxy-5,6,7,8-tetralin-2-carboxylate
[0093]
[0094] Add 1 ml of 1.0 M (R)-2-methyl-CBS-oxazoborane (1 mmol) toluene solution, 10 ml of borane-N, N-diethylaniline complex ( 10.5 mmol) toluene solution, the mixed solution was heated to 30°C, and at this moment, 30 ml of toluene-diluted 5-carbonyl-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid methyl ester (2.04 g, 10 mmol), the dropwise addition was completed within half an hour, and after continuing to stir for 2 hours at 30°C, it was cooled to room temperature, 5 milliliters of methanol solution was added to the reaction solution, and after stirring for 10 minutes, 15 milliliters of 1N hydrochloric acid aqueous solution was slowly added, and the mixture was Stir for 20 minutes, then extract with 100 ml of...
Embodiment 2
[0105] Example 2: (R)-N-{4-methyl-3-[(4-pyridin-3 base)pyrimidine-2-amino]phenyl}-5-(4-methylpiperazin-1-yl )-5,6,7,8-tetrahydronaphthalene-2-amide preparation
[0106]
[0107] Step A: Synthesis of (R)-methyl 5-hydroxy-5,6,7,8-tetralin-2-carboxylate
[0108]
[0109] Dissolve 5 carbonyl-5,6,7,8-tetrahydronaphthalene-2-carboxylate methyl ester (3.06 g, 15 mmol) in 20 ml of tetrahydrofuran solution, add active Molecular sieve 5 g, N 2 Slowly add 1.5ml of 1.0M (S)-2-methyl-CBS-oxazoborolane (1.5mmol) toluene solution dropwise at -20°C under protection, and then add borane dimethyl sulfide complex (1.14 g, 15 mmol) in 5 ml of dry tetrahydrofuran solution, after 20 minutes of dropwise addition, stirred at -15 to 20°C for 1 hour, carefully quenched the solution with 20 ml of methanol solution, raised to room temperature and continued to stir for 12 After 1 hour, 1.92 g of the product was obtained by column chromatography under reduced price vacuum drying, and the yield wa...
Embodiment 3
[0120] Example 3 (S)-N-{4-methyl-3-[(4-pyridin-3-yl)pyrimidine-2-amino]phenyl}-5-(3-methylimidazolidin-1-yl) -Synthesis of 5,6,7,8-tetrahydronaphthalene-2-amide
[0121]
[0122] Step A: Synthesis of 1-methylimidazolidine
[0123]
[0124] N-methylethylenediamine (1 g, 13.5 mmol) was added to formaldehyde (0.4 g, 13.5 mmol), potassium carbonate (6.4 g, 47.2 mmol), magnesium sulfate (5.6 g, 47.2 mmol) in 25 mL of chloroform suspension, stirred at room temperature for 18 hours, filtered, the filtrate was concentrated under reduced pressure, purified by neutral alumina column chromatography under the condition of dichloromethane:methanol=9:1 to obtain 0.81 g of product 1-methylimidazolidine, Yield 70%, MS(M+1)=87.11.
[0125] Step B: Synthesis of (S)-methyl 5-(3-methylimidazolidin-1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxylate
[0126]
[0127] Dissolve (S)-methyl 5-chloro-5,6,7,8-tetralin-2-carboxylate (2.41 g, 10 mmol) in 20 ml of DMF solvent, add potassium carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com