Etoposide compound with hydroxamic acid structure and preparation method and usage thereof
A technology of hydroxamic acid and epipodophyllotoxin, which is applied to medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., can solve the problems of easy drug resistance and single action target, and achieve good resistance The effect of tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
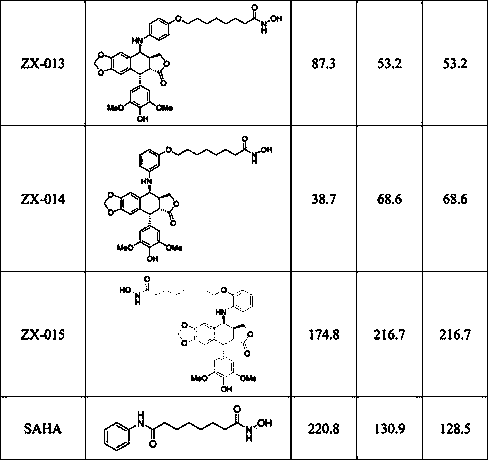
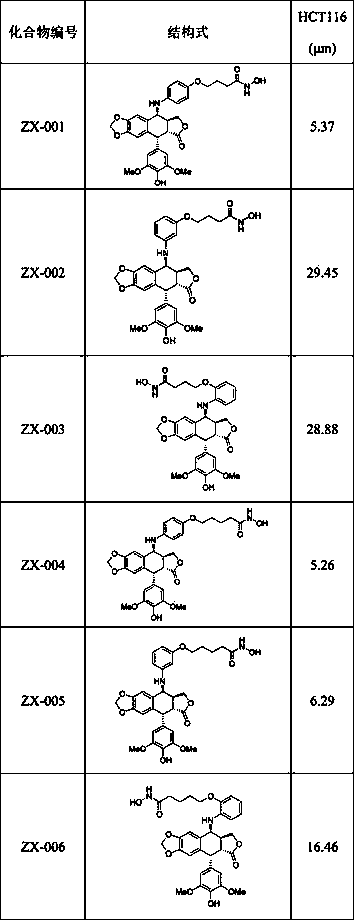
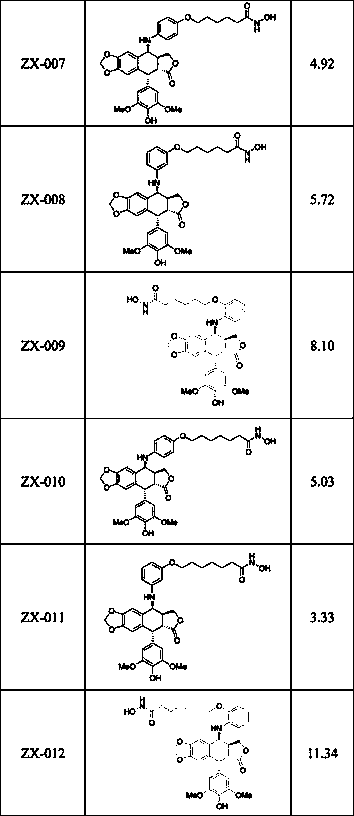
Image
Examples
Embodiment 1
[0036] 1.1 Preparation of Compound 2
[0037] Dissolve 5 g of 4-bromobutyric acid in 100 mL of methanol, add 3.3 mL of thionyl chloride dropwise at room temperature, continue the reaction for 1 hour after the drop is complete, and remove the solvent and excess thionyl chloride under reduced pressure to obtain 5.4 g of a colorless liquid . Yield 99.6%.
Embodiment 2
[0039] 1.2 Preparation of compound 3
[0040] At room temperature, add 4.52 g of potassium carbonate to the N,N-dimethylformamide solution containing 3.79 g of p-nitrophenol, stir for half an hour, add 5.4 g of methyl 4-bromobutyrate, and react at 100°C for 12 hours. After cooling to room temperature, add 100 mL of water, extract with 100 mL of ethyl acetate, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to obtain a yellow oil. Diethyl ether was added to the oil, and a large amount of solid was precipitated. After filtering, it was washed with a small amount of diethyl ether, and after drying, 3.3 g of light yellow solid was obtained, with a yield of 46.0%.
Embodiment 3
[0042] 1.3 Preparation of Compound 4
[0043] At room temperature, dissolve 2.1 g of methyl 4-(4-nitrophenoxy)butyrate in 10 mL of tetrahydrofuran, add 5 mL of 3M aqueous sodium hydroxide solution, reflux for 2 hours, and then cool to room temperature. Add 20 mL of 1M hydrochloric acid, extract with ethyl acetate, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to obtain 1.9 g of a yellow solid with a yield of 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com