Preparation method and use of cerebroside compounds
A technology of cerebrosides and compounds, which is applied in the field of medicine, can solve the problems of being unable to pass through the blood-brain barrier and having a large molecular weight, and achieve the effect of significantly mimicking nerve growth factor activity, high purity, and improving immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 compound 1 and compound 2
[0021] (1) Crushing and extraction:
[0022] After crushing 3.4 kg of Termitomyces albuminosus (Berk.) Heim, leaching with ethanol by shaking at room temperature. After suction filtration and concentration, a crude ethanol extract (593.3 g) was obtained. Subsequently, the n-butanol layer crude sample (74.9g) was obtained through solvent distribution; this part was completed with reference to the literature (Tetrahedron, 2000, 56, 5835-5841);
[0023] (2) Separation and purification:
[0024] The crude n-butanol layer was first separated by reversed-phase silica gel ODS chromatography (solvent system by volume: methanol: water=90: 10, 95: 5, 100: 0), to obtain an active component (9.9g, The elution system is methanol: water=95:5); then the component is separated by silica gel column chromatography (the solvent system is methanol by volume ratio: chloroform=5:95, 10:90, 100:0), and finally obtains The active ...
Embodiment 1
[0026] Qualitative identification of the physical and chemical characteristics and chemical structure of compound 1 and compound 2 obtained in embodiment 1:
[0027] The chemical structures of compound 1 and compound 2 were analyzed by HR MS, MS / MS, 1 H NMR, 13 C NMR, COZY, HSQC, HMBC tests determined.
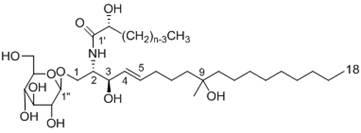
[0028] Physical and chemical properties of compound 2: amorphous powder, molecular formula is C 43 h 83 NO 10 ;
[0029] HRESI-TOF-MS m / z(M+H) + Calcd.for C 43 h 84 NO 10 : 774.6090, Found: 774.6118; FAB-MS / MS m / z 796.7 (M+Na, precursor) + , 514.7 (M+Na-fatty acyl) + , 348.6; see Table 1 for hydrogen spectrum and carbon spectrum data.
[0030] Using MS / MS technique, the length of the two carbon links of compound 2 was determined. In the MS / MS mass spectrum, the parent ion peak of compound 2 is m / z 797 (M+Na) + , the product ion peak is m / z 514 (M+Na-fatty acyl group) + , it is speculated that the aliphatic carbon chain of compound 2 contains 18 carbons (molecul...
Embodiment 3
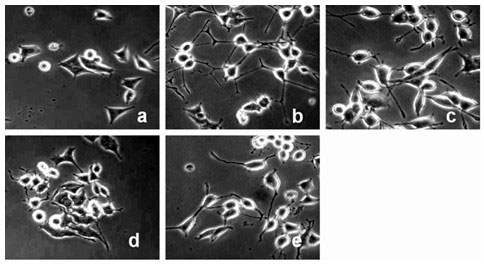
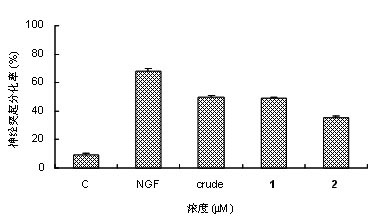
[0045] Neurogrowth-mimicking activity of cerebrosides.
[0046] PC 12 cells are cell lines cloned from rat adrenal pheochromocytoma. Under the action of NGF, PC12 cells will stop dividing, grow protrusions, and transform into neuron-like cells. Therefore, using PC 12 cells as an effective activity identification system to screen active small molecular compounds will become an effective drug for the treatment of Alzheimer's disease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com