Antifungal peptide as well as preparation method and application thereof
An antifungal peptide and antifungal technology, applied in the fields of biochemistry and biomedicine, can solve the problems of the size, composition charge and secondary structure of antimicrobial peptides, achieve low cytotoxicity, broad antifungal spectrum, overcome specific activity degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
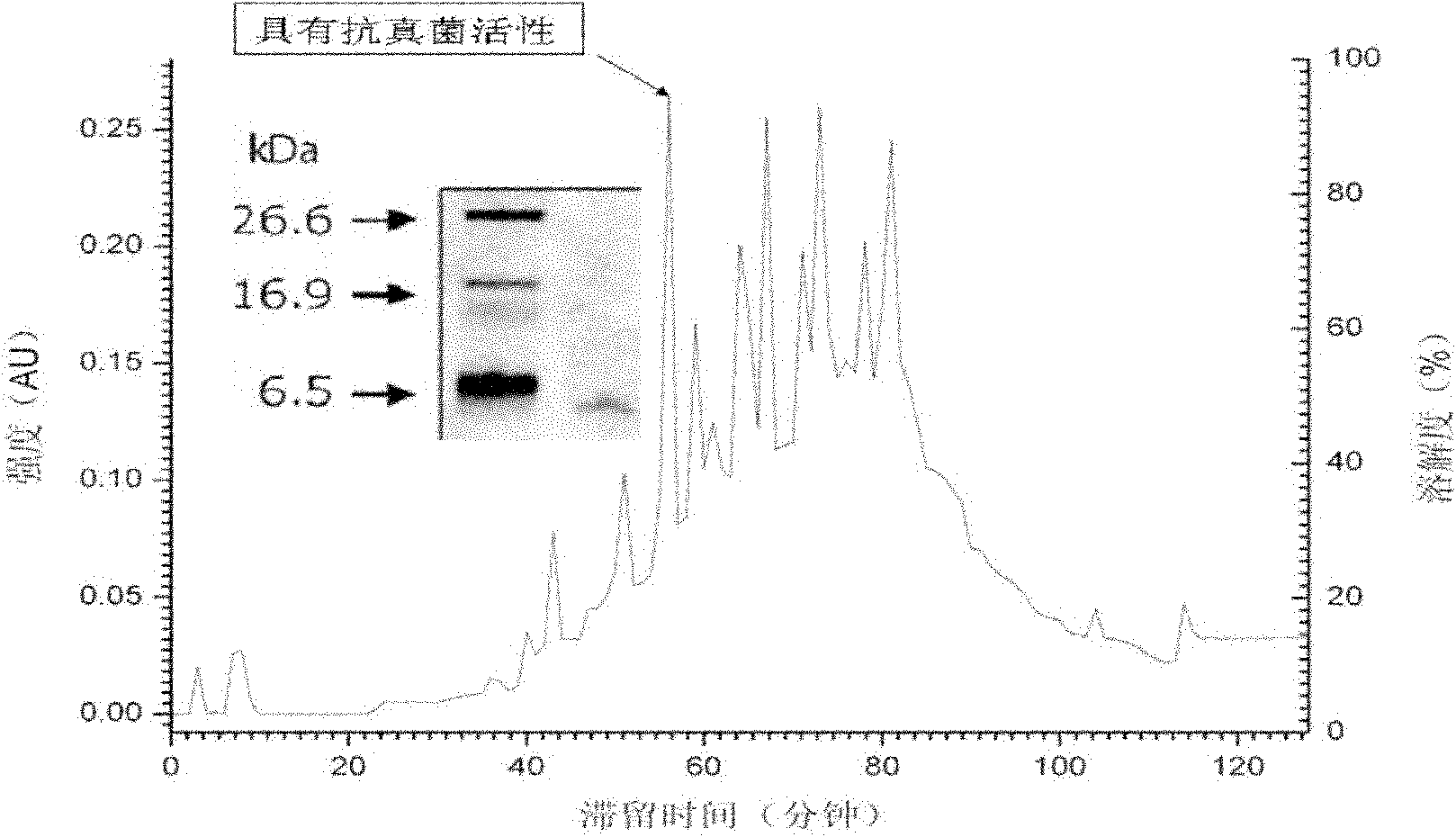
[0038] Example 1 Extraction and purification of AP polypeptide
[0039] Naturally grown bladder snails were captured from the wild and raised in the laboratory at a temperature of 15°C and a salinity of 35‰.
[0040] Collect 0.5ml of blood from each bladder snail, and add an equal volume of modified Alsever’s solution anticoagulant buffer (the buffer formula is as follows: trisodium citrate 2H 2 O 8.0g, citric acid 0.5g, anhydrous glucose 20.8g, sodium chloride 22.3g, EDTA 3.36g, distilled water 1000ml, dissolve, filter, sub-package, autoclave, 4 ℃ refrigerator for later use) mix well, put in 800g , centrifuged at 4°C for 15min; the blood cell pellet was frozen and thawed three times, then added 5 times the volume of 50mM Tris buffer (pH8.7, containing 50mM NaCl), and then homogenized with a glass homogenizer; at 10000g, 4°C Centrifuge at low temperature for 20 minutes; resuspend the pellet containing organelles with 3 times the volume of 2M acetic acid, place in a cold wat...
Embodiment 2
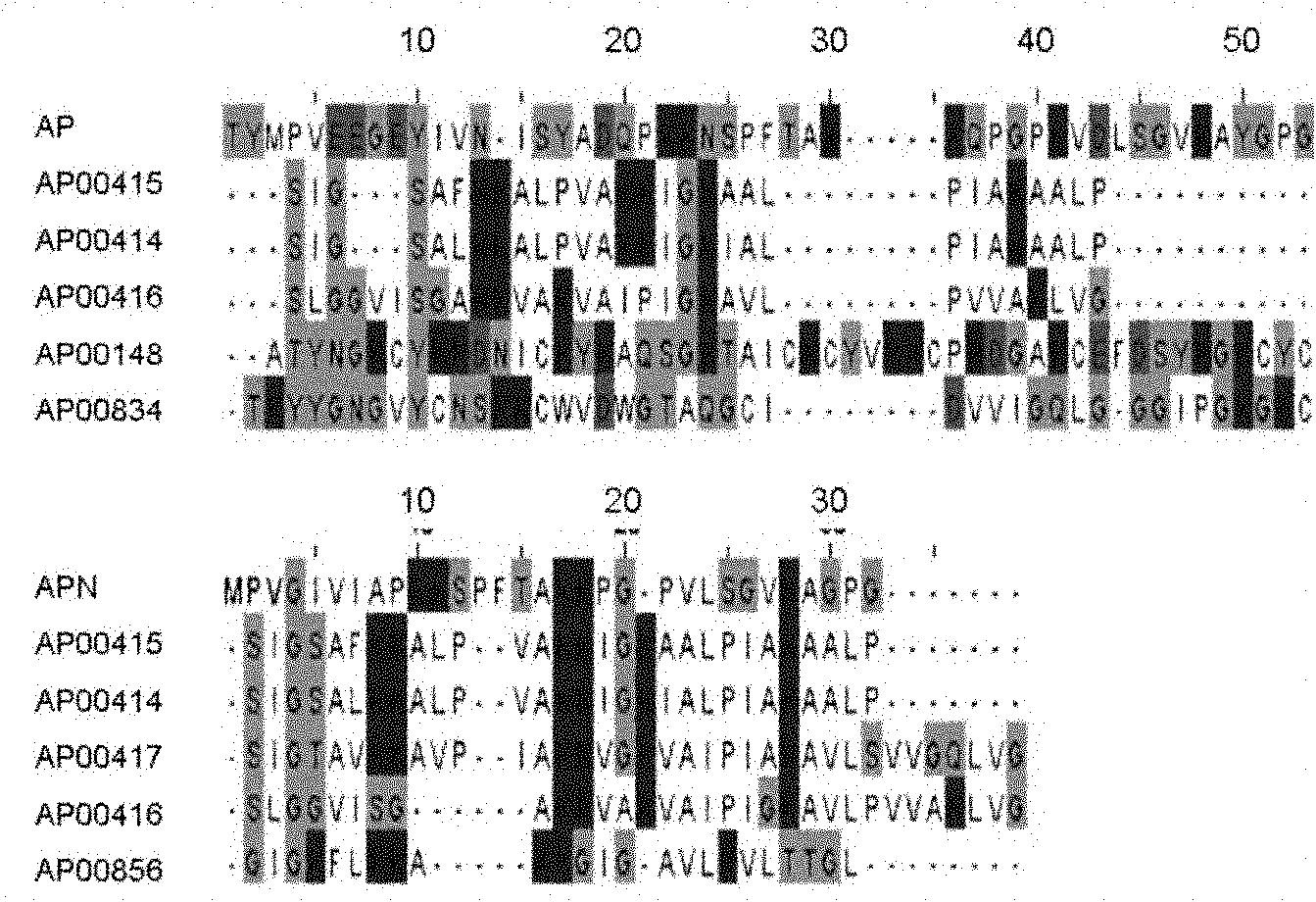
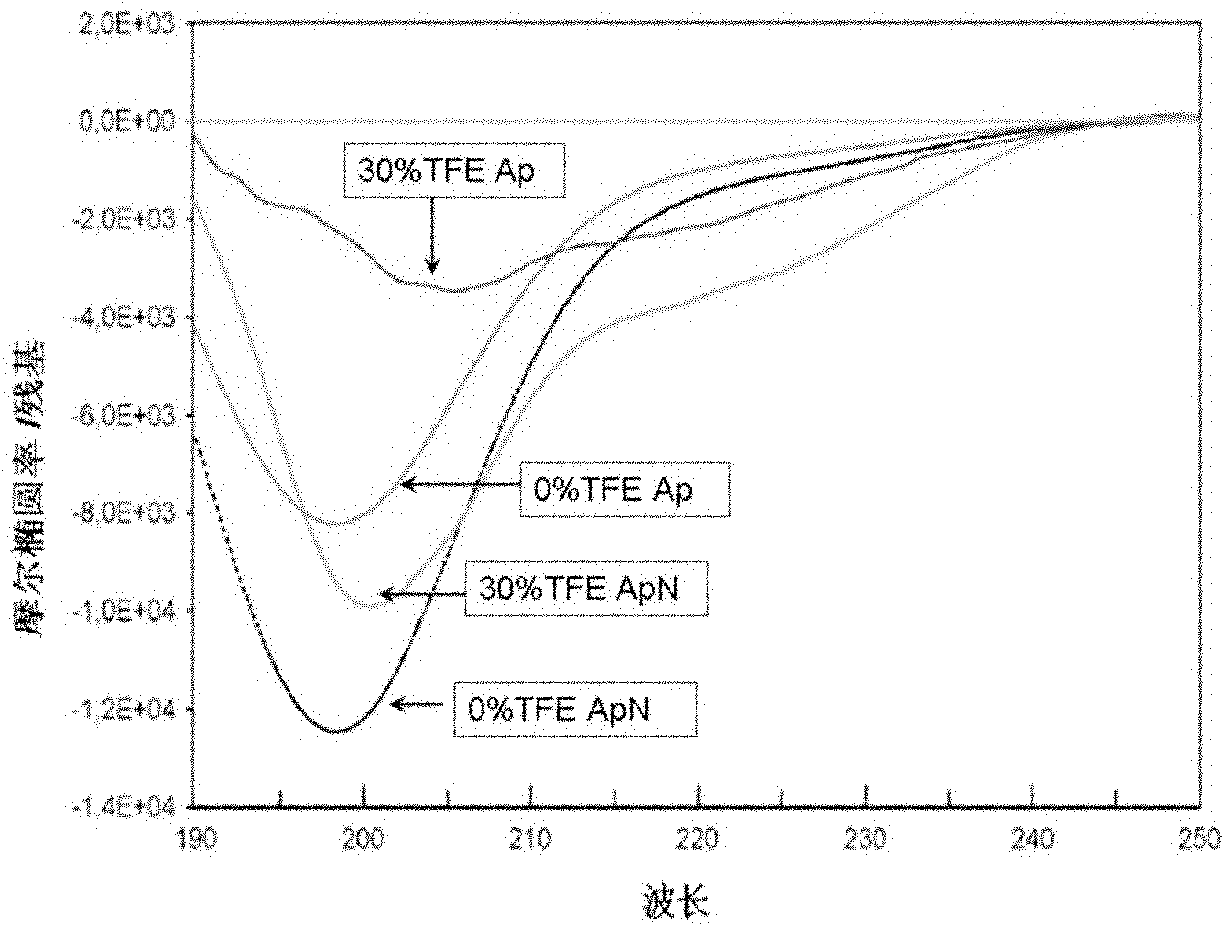
[0045] Example 2 Design of New Antimicrobial Peptide Apn
[0046] In order to improve the hydrophobicity (from 25% to 38%) and cationic surface activity (from +1 to +5) of the antimicrobial peptide, 10 hydrophilic amino acids were deleted from the N-terminus: T-1, Y-2 , Y-10, N-13, S-15, Y-16, Q-19, N-23, Q-31 and Y-44. Simultaneous deletion of five negatively charged amino acids: E-6, E-7, E-9, D-18 and D-37. The formed amphiphilic polypeptide is named Apn, which is a polypeptide different from AP, and its sequence is as follows: MPVGIVIAPKKSPFTAKKPPGVLSGVKAPGG (SEQ.ID.NO.2).
Embodiment 3
[0047] Example 3 Chemical Synthesis and Purification of Antimicrobial Peptides
[0048] The above polypeptides Ap and Apn were synthesized by standard chemical synthesis method (Lu Yingjin, Wang He. Solid-phase synthesis of antimicrobial peptides, separation and purification and study on structure-activity relationship, Acta Biological Engineering, 1997, 13(1):35-41).
[0049] After synthesis, the polypeptide was extracted with double-distilled water with 10% (v / v) acetic acid, and then purified by RP-HPLC with a purity greater than 95%. The molecular weight of the purified polypeptide Apn was determined to be 3.028kDa by mass spectrometry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com