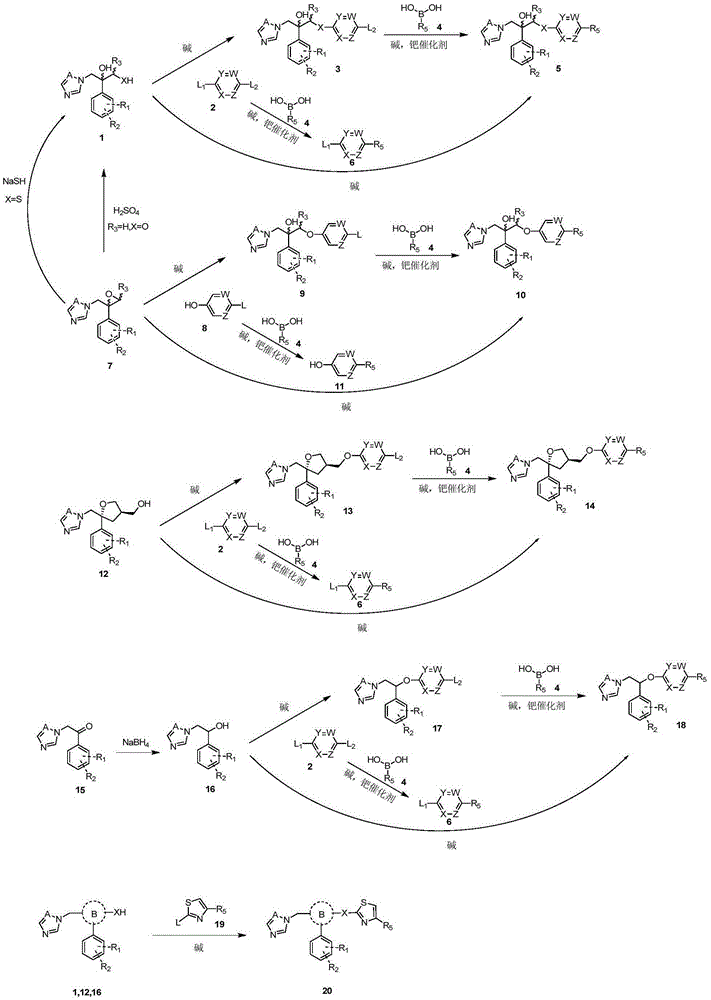
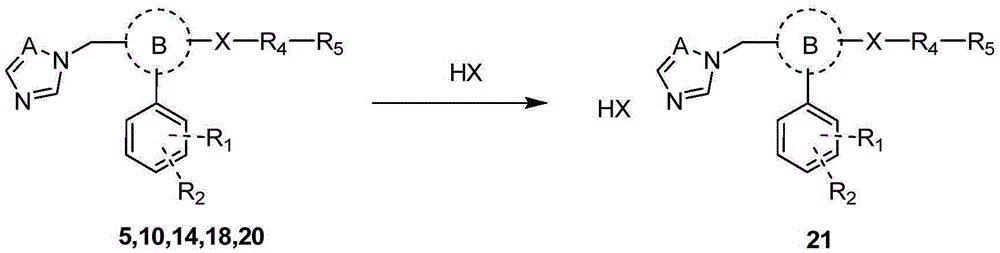
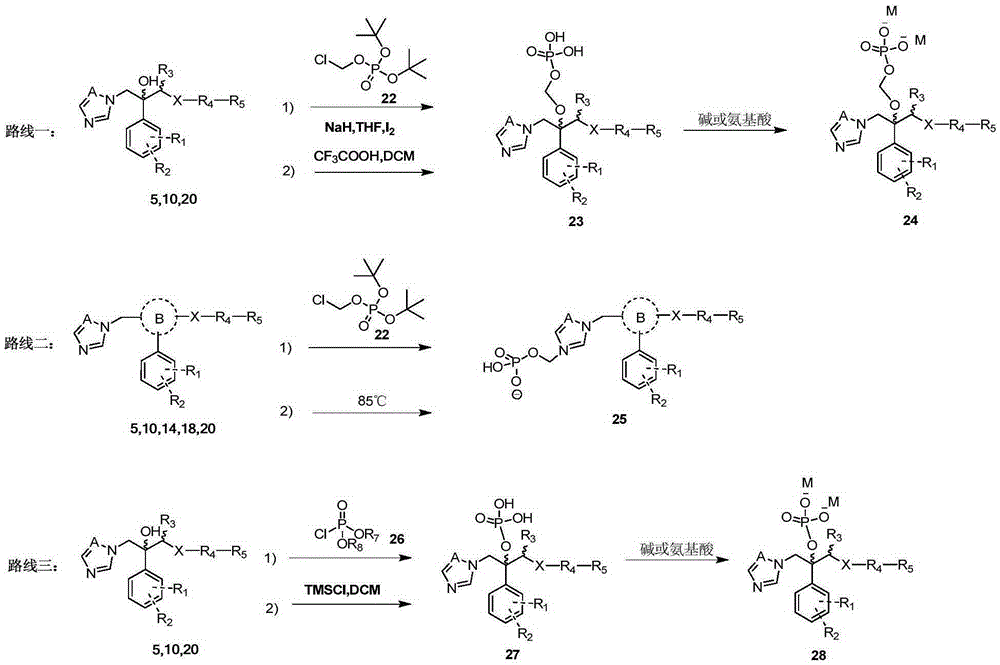
Triazole aromatic alcohol heterocyclic ether derivative and preparing method and application thereof
A kind of triazolam, aromatic heterocycle technology, applied in triazolam aromatic heterocyclic ether derivatives and preparation field thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1: 1-(5-(4-cyanophenyl)pyridyl-2-oxyl)-2-(2,4-difluorophenyl)-3-(1H-1,2,4- Synthesis of triazol-1-yl)propyl-2-ol
[0151] Step 1: Dissolve 4-cyanophenylboronic acid (5mmol), 2-fluoro-5-bromopyridine (5mmol), potassium carbonate (10mmol) and tetrakistriphenylphosphine palladium (5mol%) in toluene (20mL), Add water (2mL), react at 100°C for 5 hours, extract with ethyl acetate after the reaction is complete, wash the organic phase three times with saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate and spin to dry column chromatography to obtain 4-(6 -fluoropyridin-3-yl)benzonitrile (820 mg).
[0152] Step 2: Add 1-((2-(2,4-difluorophenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazolemethanesulfonate (50mmol) Dissolve in water, add concentrated sulfuric acid (10mL), react at room temperature for 2 hours, neutralize with sodium bicarbonate and extract with ethyl acetate, wash the organic phase with saturated aqueous sodium chloride, dry with anhyd...
Embodiment 2
[0154] Example 2: 1-(5-(4-cyanophenyl)-3-fluoropyridyl-2-oxyl)-2-(2,4-difluorophenyl)-3-(1H-1, Synthesis of 2,4-triazol-1-yl)propyl-2-ol
[0155] 4-(5,6-difluoropyridin-3-yl)benzonitrile (1mmol), 2-(2,4-difluorophenyl)-3-(1H-1,2,4-triazole -1-yl)propane-1,2-diol (1mmol) was dissolved in dimethyl sulfoxide, cesium carbonate (2mmol) was added, and reacted at 50°C for 16 hours. After the reaction was complete, the solid was filtered off, and the filtrate was spin-dried Post-column chromatography prepared the target product (110 mg).
Embodiment 3
[0156] Example 3: 1-(5-(4-difluoromethoxyphenyl)pyridyl-2-oxyl)-2-(2,4-difluorophenyl)-3-(1H-1,2 , Synthesis of 4-triazol-1-yl)propyl-2-ol
[0157] 2-fluoro-5-(4-difluoromethoxyphenyl)pyridine (1mmol), 2-(2,4-difluorophenyl)-3-(1H-1,2,4-triazole -1-yl)propane-1,2-diol (1mmol) was dissolved in dimethyl sulfoxide, cesium carbonate (2mmol) was added, and reacted at 50°C for 16 hours. After the reaction was complete, the solid was filtered off, and the filtrate was spin-dried Post-column chromatography prepared the target product (100 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com