Use of itraconazole in preparation of drug for treating multiple myeloma
A technology for multiple myeloma and itraconazole, which is applied in the field of tumor treatment and can solve problems such as refractory anemia and anti-infection treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
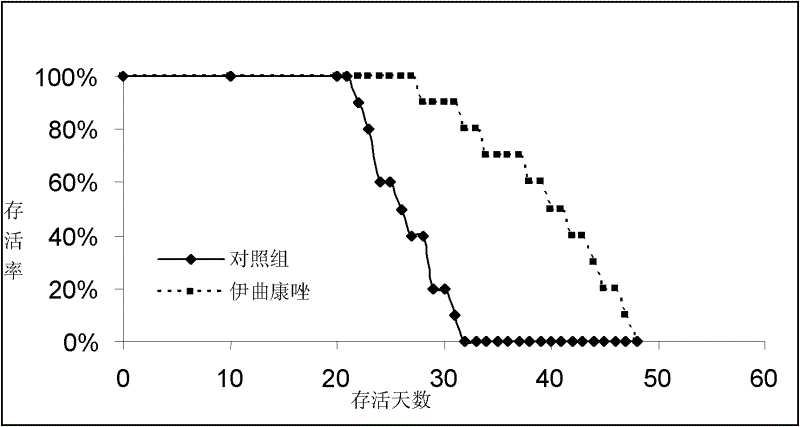
[0026] Embodiment 1, animal test
[0027] Medication method; Oral
[0028] Experimental conditions: Clean grade C57BL / KaLwRijHsd mice were used, purchased from Harlan Company in the Netherlands. C57BL / KaLwRijHsd mice were raised and tested in a clean laboratory.
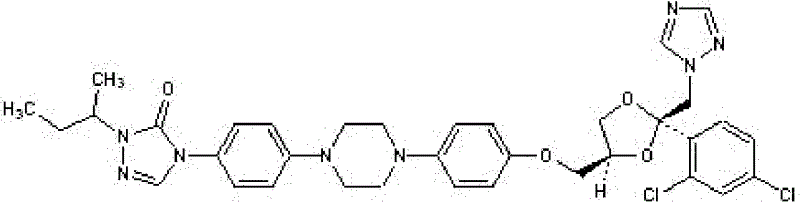
[0029] Test drug: inject itraconazole (produced by SIGMA Company of the United States), and dissolve itraconazole in dimethyl sulfoxide (produced by SIGMA Company of the United States) to prepare a solution.
[0030] Test method: Take 20 6-week-old C57BL / KaLwRijHsd mice and divide them into 2 groups, with half male and half male animals in each group, and there is no statistically significant difference in body weight among the animals in each group.
[0031] The dosing regimen for the two groups of animals is:
[0032] Control group: dimethyl sulfoxide, 0.1ml / kg body weight / day
[0033] Treatment group: itraconazole 20mg / kg body weight / day (drug concentration 4mg / ml)
[0034] One week after C57BL / KaLwRijHsd mic...
Embodiment 2
[0036] Embodiment 2 liver and kidney toxicity test
[0037] Test drug: itraconazole;
[0038] Comparing drug: bortezomib.
[0039] Test animals: mice.
[0040] Test method: refer to the "Guiding Principles of Drug Toxicology Research"
[0041] Test results: Compared with bortezomib, itraconazole has less liver and kidney toxicity, and no accidental death due to drug toxicity occurred in the mouse test.
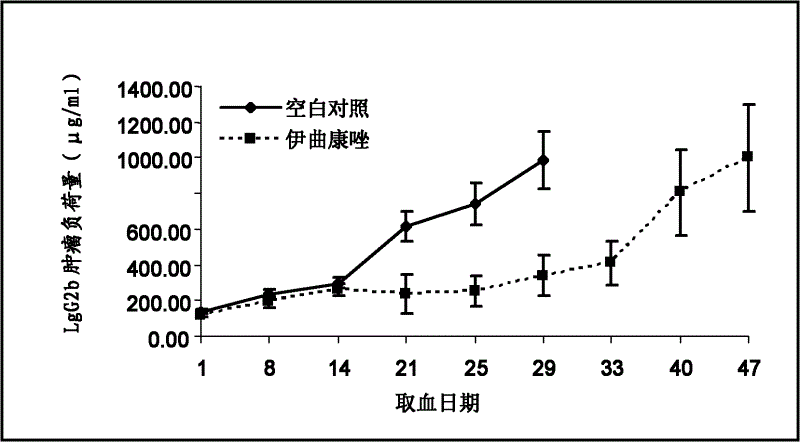
Embodiment 3
[0042] Embodiment 3 human body test
[0043] Referring to the "Technical Guidelines for Clinical Trials of Antineoplastic Drugs", select 30 patients, 20 males and 10 females, aged 30-65 years, and the observation indicators include overall survival, disease-free survival, progression-free survival, and disease progression. time to treatment failure, subject-reported outcomes and quality of life, improvement in signs and symptoms (weight gain, pain reduction).
[0044] The main symptoms were renal insufficiency in 19 cases, anemia in 17 cases, bone damage in 16 cases, fatigue in 6 cases, hypercalcemia in 2 cases, and extramedullary plasmacytoma in 1 case. Some patients have more than two symptoms. Clinical stage: 3 cases in stage I, 8 cases in stage II, and 19 cases in stage III. Bone damage was confirmed by x-ray, CT or MRI, and whole-body bone scan. The test results showed that among the 30 patients, there were 11 cases of IgG type, 10 cases of light chain type, 6 cases of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com