Precious metal-doped ZnO nanoscale particles and use of the precious metal-doped ZnO nanoscale particles as photocatalyst for unsymmetrical dimethylhydrazine wastewater degradation
A nanoparticle, unsymmetrical dimethylhydrazine technology, applied in metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, energy and wastewater treatment, etc., can solve the problem of large amount of rare earth photocatalyst, complex preparation technology, etc. The problem of high cost is to achieve the effect of complete degradation, simple preparation process and good appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
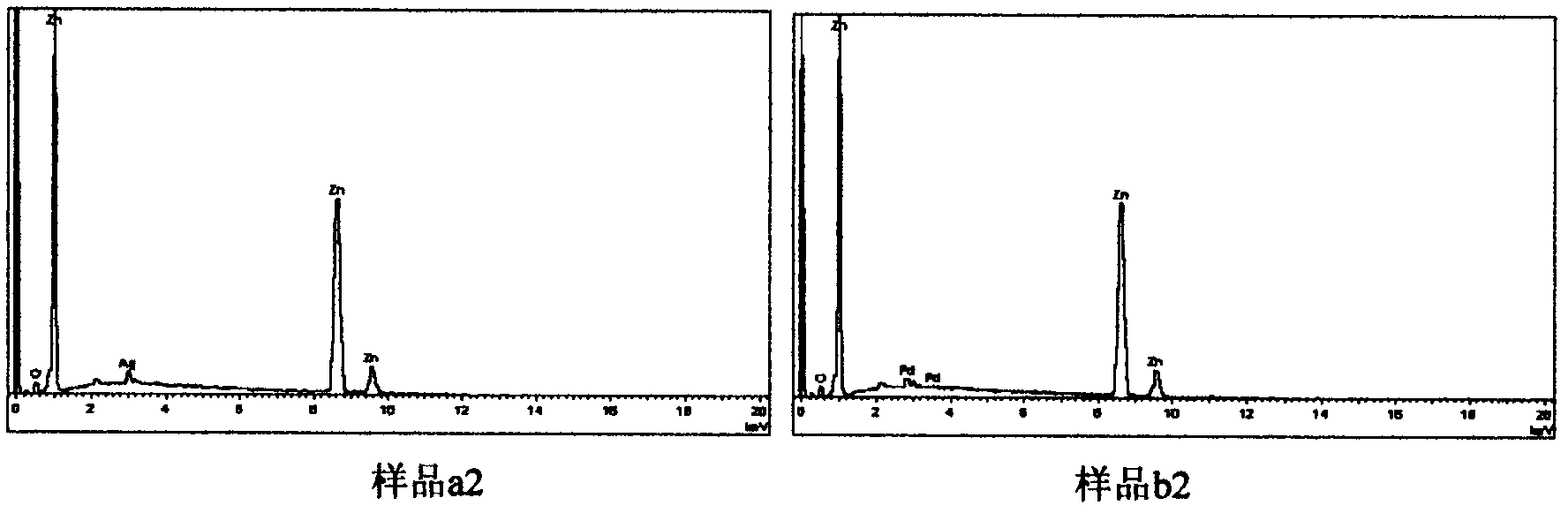
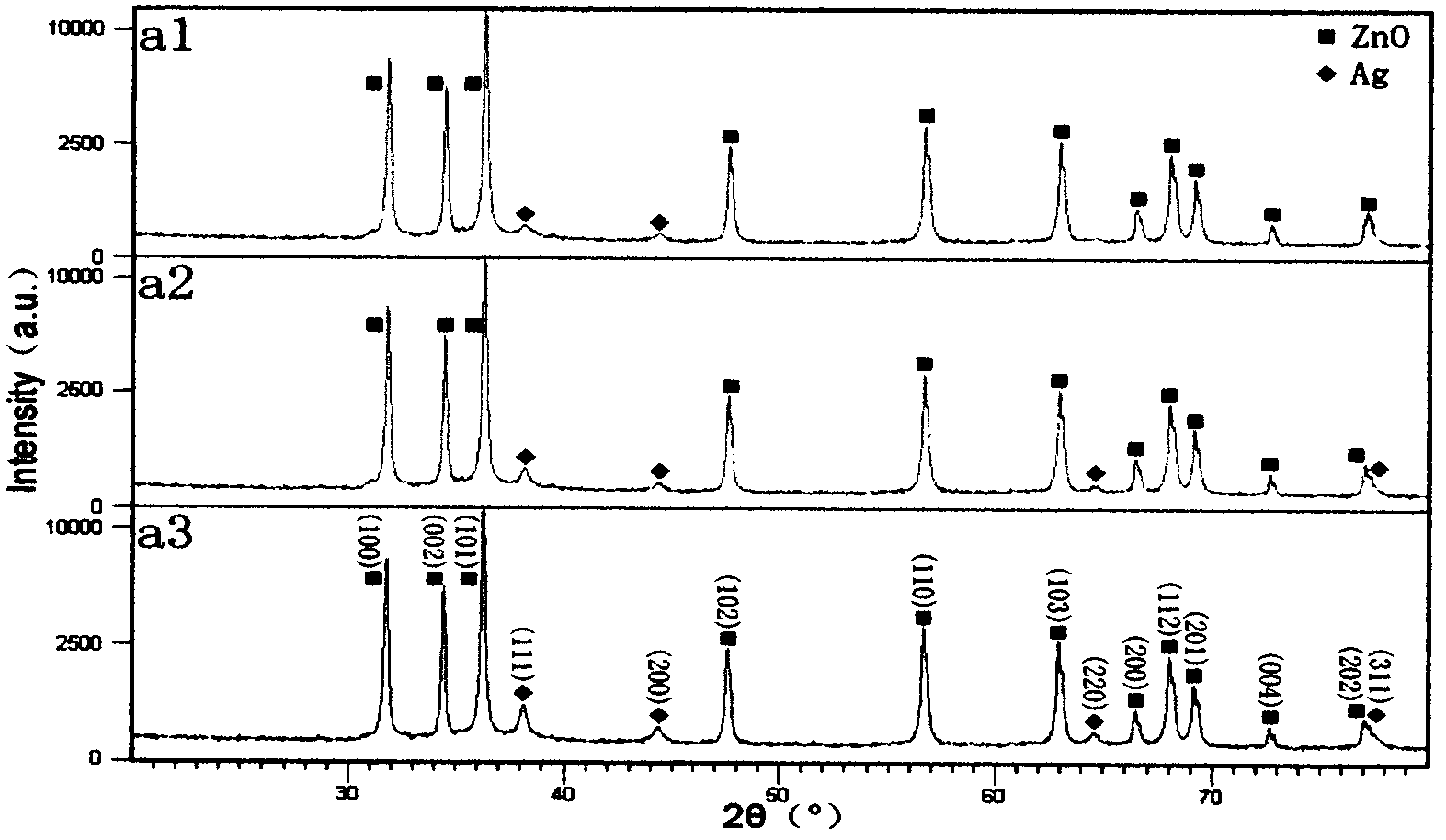
[0030] Embodiment 1: Preparation of noble metal doped ZnO nanoparticles ZnO / Ag
[0031] 1. Accurately weigh 0.2mmol of AgNO 3 Put it into a 20mL volumetric flask and dilute to the mark with distilled water to obtain 0.01mol / L AgNO 3 solution.
[0032] 2. Add 30mL of absolute ethanol to each of the three Teflon liners, and then add: a.2.5mL of 0.01mol / L AgNO 3 solution and 7.5mL distilled water; b.5mL0.01mol / L AgNO 3 solution and 5mL distilled water; c.10mL0.01mol / L AgNO 3 solution. Weigh 0.005mol of Zn(Ac) 2 2H 2 O is added to each liner to make the Zn in the solution 2+ and Ag+ The molar ratios are 100:0.5, 100:1, 100:2, respectively. Place the liner on a magnetic stirrer and stir for 10 minutes to make the Zn(Ac) 2 2H 2 O is completely dissolved, then add 0.05mol NaOH respectively, and continue to stir for 10min.
[0033] 3. Seal the Teflon liner in a high-pressure reactor, place it in a constant temperature drying oven, and react at 160°C for 12 hours.
[0034] ...
Embodiment 2
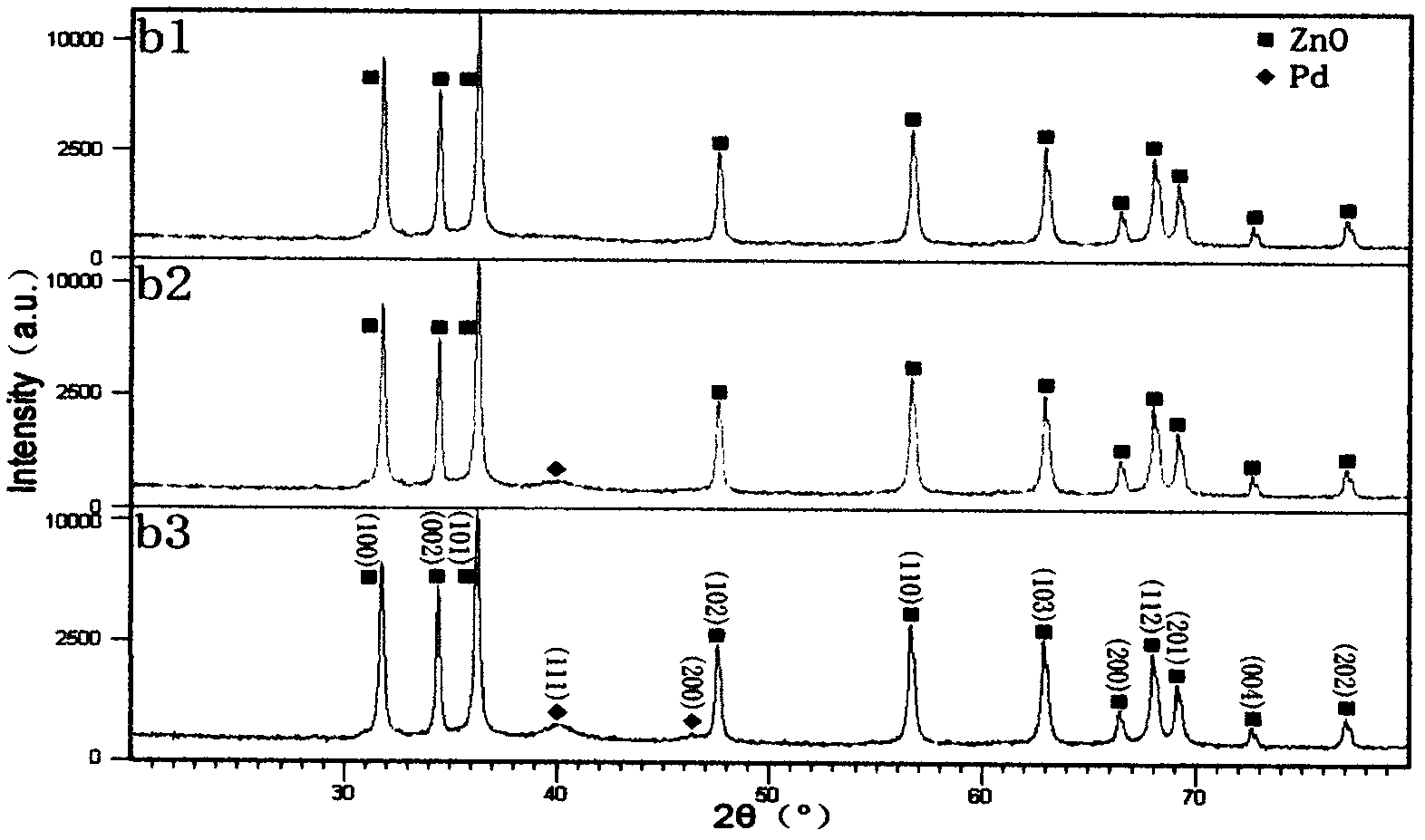
[0035] Embodiment 2: Preparation of noble metal doped ZnO nanoparticles ZnO / Pd
[0036] Due to PdCl 2 Insoluble in water, the experiment is to prepare 0.01mol / L PdCl 2 solution, using ammonia water complexation method to make PdCl 2 Form Pd(NH) with ammonia water 2 Cl 2 The complex forms a pale yellow solution in a water bath environment.
[0037] 1. Weigh 0.2mmol of PbCl 2 Add to a 20mL volumetric flask, add 0.3mL ammonia water, and dilute to the mark with distilled water. Place in a water bath at 70°C for 10 minutes to PdCl 2 Dissolve to get 0.01mol / L PdCl 2 solution.
[0038] Steps 2, 3, and 4 are the same as the preparation process of ZnO / Ag, and the final products are denoted as b1, b2, and b3.
[0039] Different from the previous photocatalytic experiments, considering that ZnO / Ag and ZnO / Pd also have strong absorption in the visible light region, their photocatalytic properties were studied by ultraviolet lamp irradiation and sunlight irradiation, respectively....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com