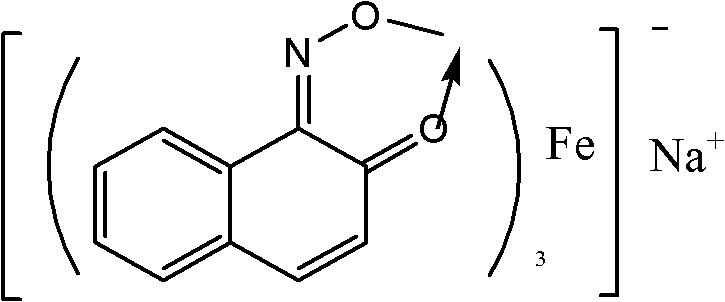
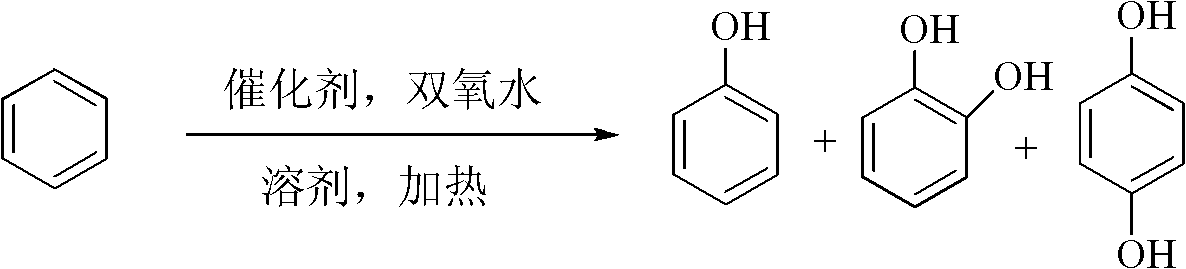
Method for preparing phenol and dihydroxybenzene by catalytic hydroxylation of benzene
A technology for benzene to catalyze hydroxyl groups and quinone, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of many reaction steps, pollution, and low utilization rate of atoms, and achieve high yield and easy The effect of industrialization and less discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 210g acetonitrile, 0.7g Pigment Green B, 10g benzene, 60g H 2 o 2 , reacted at 60°C for 5 hours. The catalyst was separated by centrifugation, and the supernatant was taken for gas chromatography analysis. After calculation, the conversion rate of benzene is 26.5%, and the yields of phenol, catechol and hydroquinone are 12.2%, 3.3% and 4.6% respectively.
Embodiment 2
[0031] Add 150g acetonitrile, 0.5g catalyst pigment green B, 10g benzene, 80g H 2 o 2 , reacted at 40°C for 7 hours. The catalyst was separated by centrifugation, and the supernatant was taken for gas chromatography analysis. The conversion rate of benzene was 27.5%, and the yields of phenol, catechol and hydroquinone were 13.4%, 4.2% and 5.6%, respectively.
Embodiment 3
[0033] Add 300g acetonitrile, 1g catalyst pigment green B, 10g benzene, 30g H 2 o 2 , reacted at 70°C for 4 hours. The catalyst was separated by centrifugation, and the supernatant was taken for gas chromatography analysis. The conversion rate of benzene was 25.7%, and the yields of phenol, catechol and hydroquinone were 14.2%, 4.6% and 5.8%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com