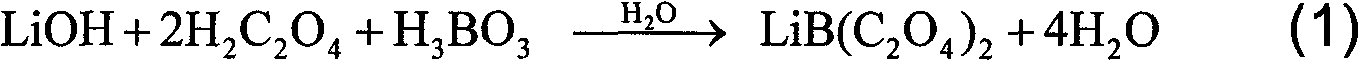Preparation method of lithium bis(oxalato)borate
A technology of bisoxalate lithium borate and lithium salt, which is applied in the field of preparation of bisoxalate lithium borate, can solve the problems of increasing the difficulty of preparation operation control, increasing the difficulty of industrialization, and complicated follow-up purification, so as to achieve easy control of operating process conditions and reduce the difficulty of control , The effect of stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The present invention is the preparation method of bisoxalate lithium borate, and its steps are:
[0022] (1) According to the stoichiometric ratio, first mix lithium salt and oxalic acid evenly, then heat at 40°C-70°C for 1-4h, and finally add boron source to the mixture, and mix all materials thoroughly;
[0023] (2) The mixture is dry-pressed under a pressure of 0.1 MPa to 10 MPa, and reacted by heating to obtain a lithium bisoxalate borate product.
[0024] According to the preparation method of lithium bisoxalate borate described above, the lithium salt used can be LiOH, or Li 2 CO 3 , or lithium alkoxide, the boron source used is H 3 B0 3 , or B 2 o 3 , the purity of the raw material is analytically pure, that is, the mass percentage is about 99.5%, or it is a higher-purity superior grade, or it is spectrally pure, or it is high-purity.
[0025] According to the above-mentioned preparation method of lithium bisoxalate borate, the heating reaction is carried ...
Embodiment 1
[0032] Weigh 7.967g H 2 C 2 o 4 2H 2 O (≥99.5%, mass percentage, the same below), 1.326g LiOH·H 2 O (≥99.5%) mixed evenly, and heated at 60°C for 2h, 1.955g H 3 BO 3 (≥99.5%) mixed evenly. The mixed mixture was pressed into tablets (tablet weight about 1.0g) under a pressure of 2 MPa (gauge pressure), and the pressed tablets were placed in a drying oven, dried in the atmosphere at 120°C for 7 hours, and then Phosphorus pentoxide was added to the drying oven, and the temperature was adjusted to 155° C. to continue drying under normal pressure for 40 hours to obtain 6.081 g of LiBOB product. The product yield is about 99.3%. Measure the oxalate content in the product with potassium permanganate titration, and measure the boron content in the product with mannitol titration, which can reach 90.53%, 5.67% (theoretical values are respectively 90.84%, 5.58%); The water content in the product was determined by non-interference Karl Fischer coulometric titration of base form...
Embodiment 2
[0034] H with a purity of 99.9% to 100.1% 2 C 2 o 4 2H 2 O replaces H in Example 1 2 C 2 o 4 2H 2 O, with H of purity ≥99.95% 3 BO 3 Replace H in Example 1 3 BO 3 , and the rest of the steps are the same as Example 1. The contents of oxalate, boron, and moisture in the obtained LiBOB product remained almost unchanged, while the contents of five metal impurity ions, calcium, magnesium, sodium, potassium, and iron, decreased to 0.00032%, 0.00048%, 0.0014%, 0.00049%, and 0.0010%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com