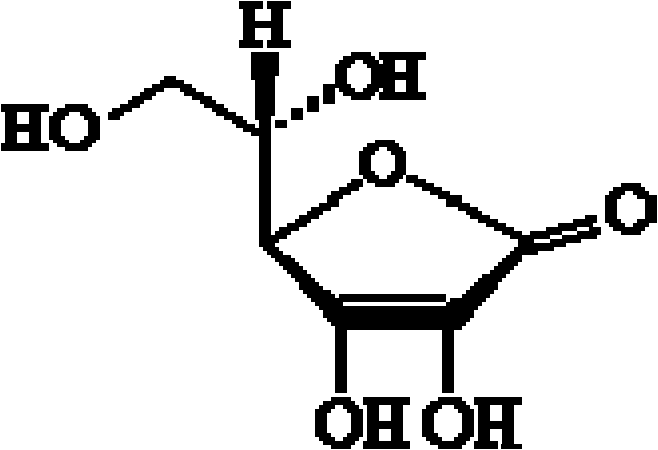
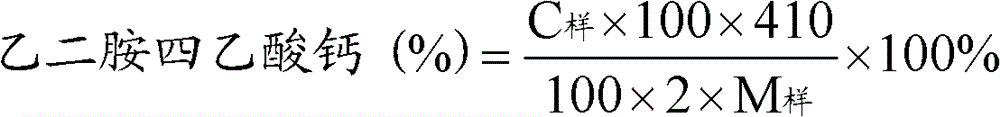A kind of preparation method of aseptic vitamin C composition and its product and application
A vitamin and composition technology, which is applied in the field of preparation of vitamin C composition, can solve problems such as difficult extraction, increased production cost, and many control conditions, so as to reduce process links and material costs, avoid degradation problems, and reduce production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0054] Add 1 kg of sodium vitamin C to 1 kg of water for injection, then add 10 g of cysteine, 50 g of calcium edetate, and 30 g of activated carbon for needles, and stir at 50±5°C for 20 minutes. Use a 0.22μm filter membrane for sterilizing filtration, inject the filtrate into a sterile crystallization tank, add 3kg of sterile ethanol solution while stirring, and lower the temperature to -4~0°C at a rate of 5°C per hour. In the process, the size of the formed crystals can be appropriately controlled to be 300-500 μm, and then kept at this temperature for 6 hours. Centrifuge and dehydrate the crystallization solution under sterile conditions, wash the crystals with a small amount (about 500g) of acetone, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±2°C, vacuum degree is 0.06-0.08MPa)4 Hours, make its moisture co...
Embodiment 2
[0059] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0060] Add 1 kg of sodium vitamin C to 1.8 kg of water for injection, then add 50 g of cysteine, 20 g of calcium edetate, and 50 g of activated carbon for needles, and stir at 45±5°C for 20 minutes. Use a 0.22 μm filter membrane for sterile filtration, inject the filtrate into a sterile crystallization tank, add 600 g of sterile isopropanol solution while stirring, and lower the temperature to -4 to 0 °C at a rate of 3 °C per hour. During this process, the size of the formed crystals can be appropriately controlled to be 300-500 μm, and then kept at this temperature for 7 hours. Centrifuge and dehydrate the crystallization liquid under sterile conditions, wash the crystals with isopropanol, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±2°C, vacuum degree is 0.06-0.08MPa) for 6 hours. The water content is less tha...
Embodiment 3
[0065] This example is used to illustrate the preparation method of the sterile vitamin C composition of the present invention.
[0066] Add 1 kg of sodium vitamin C to 3 kg of water for injection, then add 20 g of cysteine, 40 g of calcium edetate, and 100 g of activated carbon for needles, and stir for 25 minutes at 55±5°C. Use a 0.22 μm filter membrane for sterile filtration, inject the filtrate into a sterile crystallization tank, add 600 g of sterile isopropanol solution while stirring, and lower the temperature to -4 to 0 °C at a rate of 5 °C per hour. During this process, the size of the formed crystals can be properly controlled to be 300-500 μm, and then kept at this temperature for 8 hours. Centrifuge and dehydrate the crystallization solution under sterile conditions, wash the crystals with isopropanol, and then send them into a vacuum drying oven for vacuum drying (temperature is 40±5°C, vacuum degree is 0.06-0.08MPa) for 8 hours. The water content is less than 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com