Coprecipitation method for preparing methotrexate/ layered double hydroxides nanocomposite material
A nanocomposite material, methotrexate technology, applied in the field of co-precipitation method to prepare methotrexate/hydrotalcite-like nanocomposite materials, can solve the problems of difficult control of particle size distribution, easy agglomeration, etc., and achieve reliable preparation method, Good reproducibility and less contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
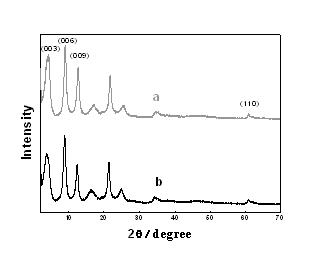
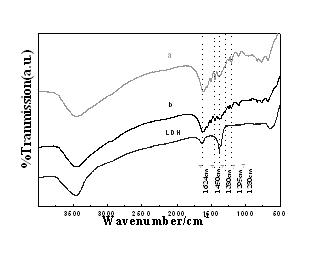
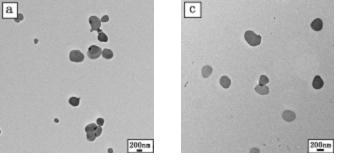
Image
Examples
Embodiment 1
[0034] 1) Weigh 0.7692 g Mg(NO 3 ) 2 ?6H 2 O and 0.5627 g Al(NO 3 ) 3 ?9H 2 O, dissolved in 20 mL PEG-400 / water solvent, where, M 2+ / M3+ =2:1, the volume ratio of PEG-400 and water is: V PEG-400 / V 水 =1:3, the metal salt mixed solution is obtained;
[0035] 2) Weigh 0.3408g of MTX, dissolve it in 15 mL of 10% NH 3 ?H 2 O solution;
[0036] 3) Add the MTX mixed solution obtained in step 2) into a closed container with a constant temperature of 60°C, blow nitrogen gas for 4 minutes, and stir at a constant speed at the same time, the stirring rate is 800r / min;
[0037] 4) Add the metal salt mixed solution obtained in step 1) dropwise to the MTX mixed solution treated in step 3) at a dropping rate of 0.05 ml / s;
[0038] 5) Add dropwise 10wt% ammonia solution to adjust the pH=9.5 of the mixed solution obtained in step 4);
[0039] 6) After the pH value of the mixed solution in step 5) is constant, start timing. After 0.5 hours, cool the mixed solution to room tempera...
Embodiment 2
[0046] 1) Weigh 1.3387 g Zn(NO 3 ) 2 ?6H 2 O and 0.5627 g Al(NO 3 ) 3 ?9H 2 O, dissolved in 25 mL PEG-300 / water solvent, where, M 2+ / M 3+ =3, the volume ratio of PEG-300 and water is: V PEG-300 / V 水 =1:2, the metal salt mixed solution is obtained;
[0047] 2) Weigh 0.5453 g of MTX and dissolve it in 10 mL of 5% NaOH solution;
[0048] 3) Add the MTX mixed solution obtained in step 2) into a closed container with a constant temperature of 70°C, blow nitrogen gas for 2 minutes, and stir at a constant speed at the same time, the stirring rate is 600r / min;
[0049] 4) Add the metal salt mixture obtained in step 1) dropwise to the MTX mixed solution treated in step 3) at a dropping rate of 4 ml / s;
[0050] 5) Add dropwise 5% NaOH solution to adjust the pH=8 of the mixed solution obtained in step 4);
[0051] 6) After the pH value of the mixed solution in step 5) is constant, start timing. After 3 hours, cool the mixed solution to room temperature and pour it into a ce...
Embodiment 3
[0055] 1) Weigh 1.538g Mg(NO 3 ) 2 ?6H 2 O and 0.6060 g Fe(NO 3 ) 3 ?9H 2 O, dissolved in 15 mL PEG-600 / water solvent, where, M 2+ / M 3+ =4, the volume ratio of PEG-600 and water is: V PEG-600 / V 水 =1:4, the metal salt mixed solution is obtained;
[0056] 2) Weigh 0.2045 g of MTX and dissolve it in 25 mL of 20% methylamine solution by mass percent;
[0057] 3) Add the MTX mixed solution obtained in step 2) into a closed container with a constant temperature of 50°C, blow nitrogen gas for 7 minutes, and stir at a constant speed at the same time, the stirring rate is 1000r / min;
[0058] 4) Add the metal salt mixture obtained in step 1) dropwise to the MTX mixed solution treated in step 3) at a dropping speed of 2 ml / s;
[0059] 5) Add 20% methylamine solution dropwise to adjust the pH of the mixed solution obtained in step 4) to 11;
[0060] 6) After the pH value of the mixed solution in step 5) is constant, start timing. After 5 hours, cool the mixed solution to ro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak intensity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com