Method for preparing tertiary alcohol by means of Grignard reaction
A technology of Grignard reaction and tertiary alcohol, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of expensive additives, unfriendly environment, and reduced drug efficacy, and achieve simple and easy reaction operation Easy to use, low price, and the effect of reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of n-BuMgBr Grignard reagent
[0023] In a 250mL three-necked flask, add 2.55g (105mmol) magnesium chips, add 3 iodine particles (as an initiator), and pass through nitrogen protection, add 10mL dry THF to immerse magnesium chips, start stirring, add 1.0mL n-BuBr , heating to initiate the reaction, causing the color of the iodine particles to fade, THF reflux, the surface of the magnesium chips is shiny, and the magnesium chips gradually become less. After initiation, stop heating, slowly add n-BuBr THF solution (containing n-BuBr 9.8mL, dry THF solution 90mL) dropwise, heat to 65°C and reflux for 1h after dropping. The titration of the Grignard reagent refers to the acid-base titration method, and the concentration of n-BuMgBr synthesized according to this operation step and reagent dosage is about 1mol / L.
Embodiment 2
[0024] Embodiment 2: the synthesis of 2-phenyl-2-hexanol
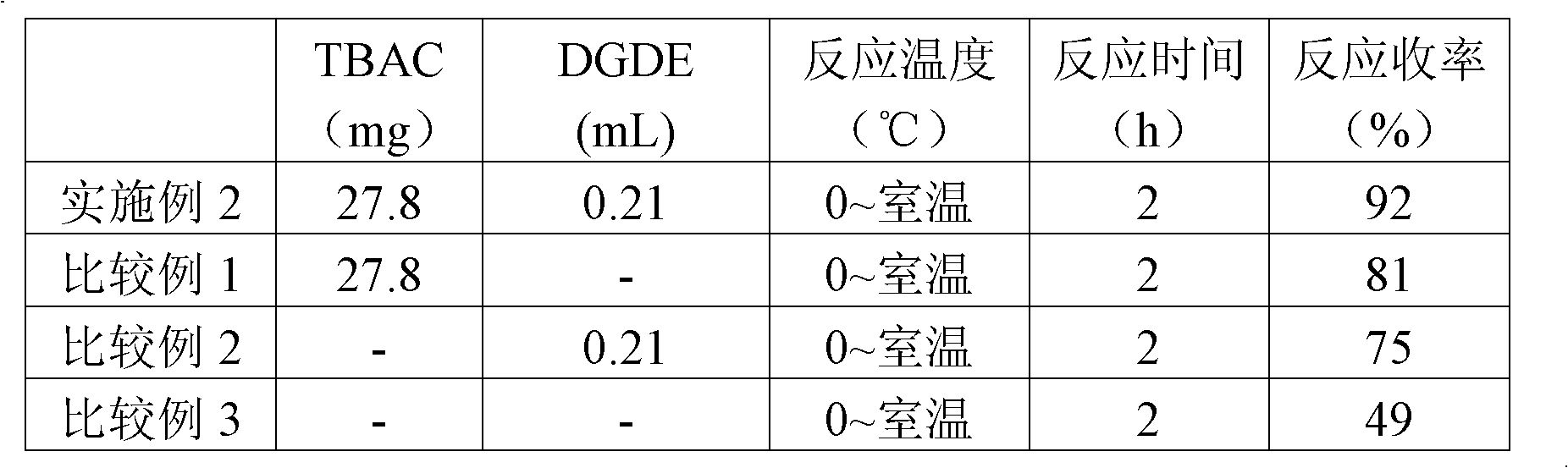
[0025] In a 50mL round-bottomed flask, add the quaternary ammonium salt tetrabutylammonium chloride (TBAC) (27.8mg, 0.1mmol), fill it with nitrogen and seal it, and slowly add the Grignard reagent n-BuMgBr (1.5mL, 1.5mmol , 1.0M in THF), stirred at room temperature for 10min, then added diethylene glycol dimethyl ether (DGDE) (0.21mL, 201mg, 1.5mmol), continued to stir at room temperature for 30min, then gradually cooled from room temperature to 0-5°C , a solution of acetophenone in tetrahydrofuran (120 mg, 1.0 mmol, 1.0 M in THF) was added slowly.
[0026] After the dropwise addition, the reaction solution was gradually raised to room temperature and stirred for 2 hours. After the reaction was completed, 5 mL of saturated NH 4 The reaction was quenched with aqueous Cl solution, extracted with ethyl acetate (10 mL×3), washed with saturated sodium chloride (5 mL), dried over anhydrous sodium sulfate for 20 min, filtere...
Embodiment 3
[0035] Embodiment 3: the synthesis of 3-methyl-2-phenyl-2-butanol
[0036] Replace n-BuMgBr with i-PrMgBr, and the synthesis process is the same as in Example 2. Yield: 82%. The product is a colorless oily liquid. NMR analysis (Burker AVANCE 400 spectrometer): 1 H NMR (400MHz, CDCl 3 , TMS) δ0.82(d, J=6.9Hz, 3H), 0.90(d, J=6.9Hz, 3H), 1.53(s, 3H), 1.73(s, 1H), 2.03(septet, J=6.9 Hz, 1H), 7.20-7.45 (m, 5H); 13 C NMR (100MHz, CDCl 3 )δ 17.2, 17.4, 26.7, 38.6, 76.7, 125.3, 126.4, 127.9, 147.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com